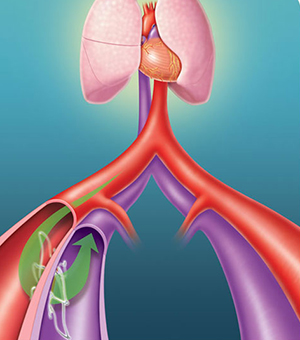
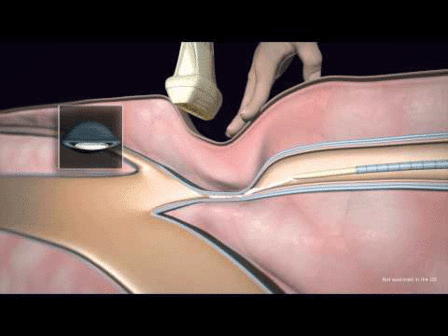
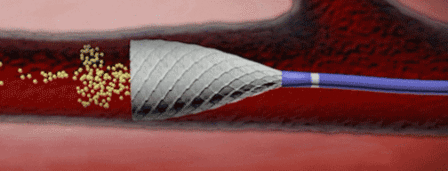
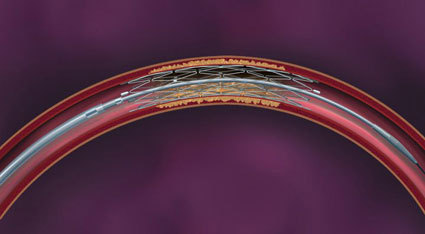
“Accurate diagnosis, control of ischaemia, and control of infection are essential in order to reduce the number of avoidable amputations,” said Michael E Edmonds, consultant in diabetes, London, UK, and one of the three programme directors of ilegx.
Gunnar Tepe, interventional radiologist from Rosenheim, Germany, and another ilegx programme director, said, “Early referral for revascularisation is a critical ingredient to save legs.” Dieter Mayer, vascular surgeon, wound care specialist, Zurich, Switzerland, and the third ilegx programme director, added, “The interdisciplinary team approach is key to bringing this about.”
These statements echoed the fundamental message of ilegx which is to create awareness of the different causes of leg/foot tissue loss and to encourage the various specialties to work together.
“In a multidisciplinary team, it does not matter who the leader is, as long as there is a flagbearer for saving legs” said Matthias Augustin, Hamburg, Germany, one of the faculty at the conference.
It is widely recognised that an interdisciplinary team is much more effective in the management of diabetic foot ulcers with a view to reducing amputations and Augustin was one of the many specialists of different nationalities including interventionalists, vascular surgeons, radiologists, diabetologists, dermatologists and would care specialists who congregated in Munich on 13-14 October to share expertise on leg/foot tissue loss.
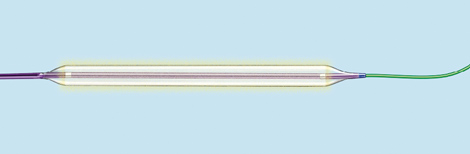
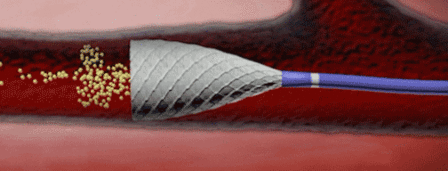
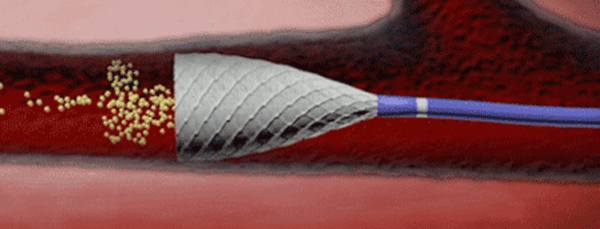
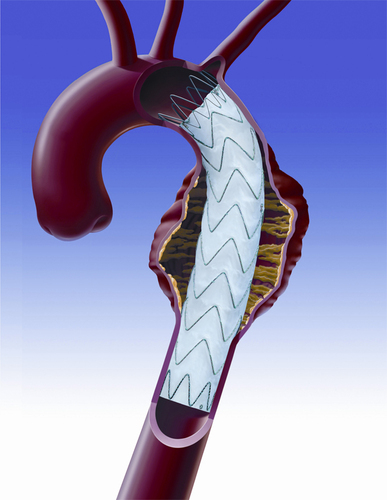
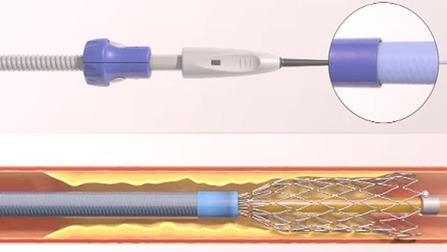
To stent or not to stent, that is the question
“Even the industry has recognised that no stent is a good stent for the leg,” said Gunnar Tepe, who was debating the topic ‘Balloon angioplasty is enough’. He was alluding to bioabsorbable stents, which are still in early development and conceded that these may be the future of stenting technology.
Speaking for the motion, Tepe told ilegx delegates in no uncertain terms to say “no” to stents as first line treatment.
“Stents are very expensive, for technical reasons only short spot stenting is feasible, there is no increased patency with stenting and if at all there is, it usually has no clinical relevance,” he said.
Speaking on balloon angioplasty as a sole treatment option, Tepe said that it showed “excellent” results. He also said that with stenting, there was often a new problem created, that of in-stent restenosis.
Overall, Tepe argued that it “much too early to say stents have to be used. In addition, bare metal stents vs. drug eluting stents have shown no significant difference.”
Thomas Zeller, Tepe’s opponent in the debate said, “Balloon angioplasty alone is not enough in below-the-knee revascularisation, especially in lesions smaller than 10cm.
For example, in a focal lesion across the knee, plain balloon angioplasty might be associated with insufficient primary success and high restenosis rate; atherectomy could be potentially associated with an increased risk of perforation and so a self-expanding low profile nitinol stent, might be the answer,” he said.
Zeller quoted Gunnar Tepe’s own paper “Self- expanding stents for treatment of infragenicular arteries following unsuccessfull balloon angioplasty,” which was published in European Radiology back at him.
He said data from the paper showed that when 24 stents were implanted in 20 such arteries, at six months follow-up of all patients, there was 100% technical success, 88% clinical imporvement and an 18% restenosis rate.
However, Tepe had pre-empted this argument for ilegx delegates by saying that this study had a small cohort, and had showed a lot of restenosis.
Endovascular therapy and surgery are complementary, not exclusive
In the debate between Dierk Scheinert, Leipzig, Germany, and Gerhard Rumenapf, Speyer, Germany, on the topic “Endovascular therapy is better than surgery,” ilegx delegates got to know first-hand how while results from historical studies and the idea that surgery and endovascular procedures were exclusive were one thing, reality on the ground is quite another.
Scheinert said that while “My opponent will say that surgery is the only viable and durable option for patients with long lesions and long total occlusions, as shown in some past studies, but in reality decisions are based on many factors. Comorbidities often need to be considered,” he said.
He put the results of past trials like BASIL which showed that outcomes and advantages of surgery were similar to endovascular therapy in terms of patients remaining asymptomatic in perspective. Scheinert told delegates that at the time of BASIL,”We had no appropriate stents and we had to use coronary stents off-label.” Nowadays we have a wide choice of self-expanding and balloon-expandable stents, but the restenosis rate is still too high, he admitted.
“Less is definitely more in the treatment of critical limb ischaemia,” said Scheinert referring to any less-invasive treatment options compared with the invasive nature of bypass. “New devices have helped to improve success rates of endovascular therapy and we are attempting to achieve what we must achieve, which is avoiding amputations,” he said.
On the opposing side of the debate, Rumenapf said “I think endovascular therapies and surgery should complement one another, not replace one another.”
“The Charing Cross Consensus, the International Diabetic Foot guidelines and the New England Journal of Medicine national guidelines all say the same thing. First, the treatment of these lesions should be interdisciplinary, second, if the short term and long term symptomatic improvement is expected to be equivalent, endovascular techniques should be used first. It is difficult to talk against this.”
He told ilegx delegates, “I cannot say anything against endovascular therapy if it is reasonably done. So before asking what remains for vascular surgeons or what is better, consider that the vascular surgeon can offer both techniques. The number of patients is rising but the number of bypass procedures remains constant,” he said.
After revascularisation, what next? Regular follow up!
“Regular follow-up and self surveillance are vital for critical limb ischaemia patients who have undergone revascularisation,” Ulrich Hoffman, Munich , Germany, told ilegx delegates .
He was talking on aspects of managing patients with critical limb ischaemia beyond revascularisation. Hoffman stated that such patients had high risk of restenosis . “These patients should be seen at least twice with physicians focussing on interval history, physical examination, ABI and imaging. We should also give clear instructions to patients on how to perform self-surveillance,“ he said.
Hoffman told ilegx delegates how important it was to revascularise critical limb ischaemia patients even if there were increased procedural risks, doubtful (long-term) success and high procedural costs. “This is because critical limb ischaemia is associated with high mortality, morbidity and costs,” he said.
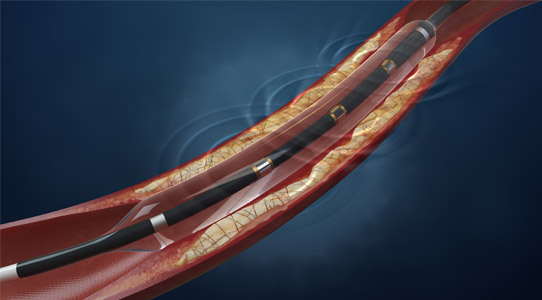
Now angioplasty can treat femoral lesions once considered “impossible”
In an ilegx session that left interventionalists gasping at how far you could go with angioplasty, Lanfroi Graziani, succeeded in demonstrating how exactly to push the boundaries with this endovascular procedure.
How far can you really go with angioplasty? How do you go where no interventionalists usually go? These were the questions Lanfroi Graziani, Brescia, Italy addressed in a session titled “Angioplasty pushed to its limits.”
He told ilegx delegates, “Transluminal balloon angioplasty remains our best option in treating patients with critical limb ischaemia. Due to improvements in techniques and devices, we can now treat femoral lesions once considered impossible to treat – but specific training is required.
Graziani highlighted that the use of sophisticated instruments has enabled achieving good results in patients, even in the treatment of extreme lesions in dialysed subjects with critical peripheral ischaemia.
Angioplasty is a well-established technique in peripheral arterial disease and critical ischaemia, particularly in the case of the lower limb arteries and extremely calcified femoral popliteal segments,” he said.
Graziani said that in his institution, psoralen with long wavelength ultra violet radiation after balloon angioplasty is the first line treatment. “We believe in transluminal balloon angioplasty as it is a minimally-invasive, repeatable technique and a low-cost procedure,” he said.
In integrated extreme intervention, the lesion is usually approached from the femoral artery going down to the foot. “This kind of technique represents 90% of procedures used in critical limb ischaemia cases”, Graziani stated. “Stenting in the fem-pop segment and balloon angioplasty are combined together for the best revascularisation.”
Graziani has said that three tips to optimise the result of balloon angioplasty would be to ensure that 1 ) there is prolonged balloon inflation (>180 sec), 2) gradual high-pressure balloon dilatation and 3) dilatation using a correct balloon size.
The second annual ilegx symposium took place in Munich, Germany on 13-14 October, 2009 and was attended by around 250 delegates for the second year running.