By Claudio Schönholz
Carotid artery angioplasty with stenting has become a viable alternative treatment for carotid artery occlusive disease. Periprocedural embolization is recognised as a factor that prevents carotid artery stenting from achieving optimal procedural safety. The main risk associated with carotid artery stenting is periprocedural stroke or asymptomatic brain infarction resulting from embolization of debris during predilatation, stenting, and postdilatation of the carotid artery stenosis. The goal of cerebral protection devices is to prevent or minimise embolization to the brain. This is the justification for the employment of a variety of cerebral protection devices, including proximal and distal balloon occlusion, distal filters, and proximal protection with flow reversal. Embolization has been shown to occur during every stage of carotid artery stenting, but embolic protection starts once the cerebral protection device is deployed and finishes when the device is retrieved. This means that embolization can still occur during the initial placement of the guide catheter in the artery and can also be seen after the procedure has been completed. Low major adverse event rates require a careful technique and limitation of instrumentation to minimise embolization during the initial phase of the procedure with administration of dual-antiplatelet medication, statins, and the appropriate use of stents of adequate size and cell configuration to sufficiently manage post-procedure embolic risk.
The flow reversal system is an endovascular tool which emulates the protection strategies of carotid endarterectomy by; establishing protection prior to crossing the lesion, inclusion of a shunt, and flushing of the lesion with flow reversal prior to re-establishing antegrade flow. To assess both symptomatic and asymptomatic cerebrovascular embolic events in real time, transcranial Doppler was utilised in a study at the Medical University of South Carolina while patients underwent carotid artery stenting. A group of patients was protected using various distal filters that had been approved by the US Food and Drug Administration (FDA) with the second group receiving neuroprotection via the Gore Flow Reversal System. Transcranial Doppler signal recordings were evaluated for three stages of the procedure; protection device deployment, stent delivery including pre and post-dilatation, and protection device removal. Patients undergoing carotid artery stenting using the Gore Flow Reversal System demonstrated significantly less average total microembolic signals counts compared to procedures using distal filter devices (431.3±65.4 vs. 116.3±20.8, n=14; p<0.001).
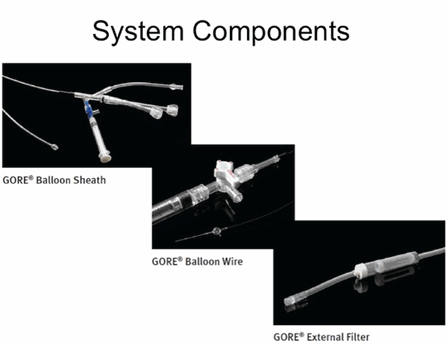
Proximal protection with the Gore Flow Reversal System utilises three main components: the Gore Balloon Sheath, the Gore Balloon Wire, and the Gore External Filter. The Gore Balloon Sheath, which contains a compliant occlusion balloon, is placed in the distal segment of the common carotid artery to temporarily stop antegrade blood flow. The Gore Balloon Wire component is placed in the external carotid artery to prevent collateral flow from the external carotid artery to the internal carotid artery. The Gore External Filter is placed outside the patient between the Gore Balloon Sheath and the venous sheath placed in the femoral vein to create an arteriovenous shunt. Flow reversal is initiated by inflating the balloons in the common carotid artery and external carotid artery and allowing the difference between the arterial and venous pressures to drive retrograde blood flow in the internal carotid artery through the lumen of the Gore Balloon Sheath and on through the Gore External Filter into the venous circulation. When flow is reversed at the level of the lesion, any particle from the plaque or clot that becomes dislodged during the procedure will follow flow away from intracranial circulation preventing a stroke or silent infarction.
In conclusion, analysis of the study data suggests that patients undergoing carotid artery stenting under flow reversal with the Gore Flow Reversal System have significantly fewer microembolic signals than patients protected with filter devices.
Some clinical studies confirmed the level of cerebral protection during carotid artery stenting procedures by showing a very low incidence of stroke, including challenging patient subgroups (ie. symptomatic and octogenarian patients). The multicentre EMPIRE (Embolic protection with reverse flow) study evaluated 30-day outcomes in high surgical-risk patients with carotid artery stenosis treated with carotid artery stenting using the Gore Flow Reversal System. The trial included 245 patients of which 32% were symptomatic. The 30-day stroke and death rate was 2.9%. Six patients showed intolerance to flow reversal (2.4%).
Another study, the multicentre European study, recently published by Nikas et al, reports on 122 patients from 10 high volume carotid artery stent European centres (including 28% symptomatic) that underwent carotid artery stenting using the Gore Flow Reversal System as neuroprotection. The 30-day death/stroke rate, as well as the incidence of myocardial infarction was 1.6%. Neither death nor myocardial infarction was observed within 30 days. Intolerance was seen in only 1.6% of patients. These results represent some of the lowest complication rates to be reported in studies assessing efficacy of embolic protection devices.
Claudio Schönholz is professor of Radiology, Medical University of South Carolina, USA













