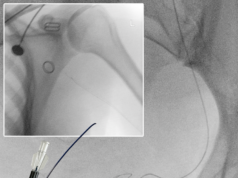
ev3 announced that a voluntary Class I recall of the TrailBlazer Support Catheter was completed in December 2009, and all affected units are in the company’s possession.
On January 5, 2010, the US Food & Drug Administration (FDA) disclosed the voluntary recall of the TrailBlazer Support Catheter. The voluntary recall was initiated in November 2009 after the company received reports of cracking on the device near the marker band from physicians during the initial limited market release of the device in the USA. The action was reported to the FDA, and 28 affected accounts were notified by physician letter. The recall of approximately 350 individual catheters was completed and a final closure report was sent to the FDA in December 2009.
No other ev3 devices were involved in this action. In December, ev3 launched a redesigned TrailBlazer Support Catheter that is currently available globally.