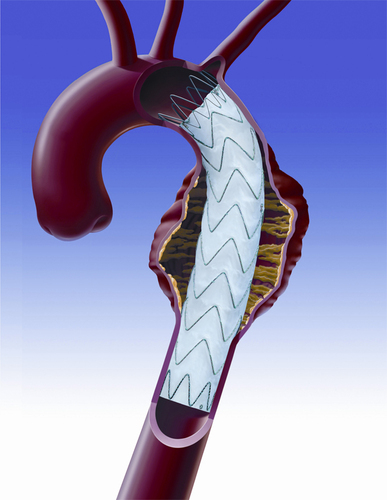
Medtronic has received US FDA approval for use of the Valiant Captivia Thoracic Stent Graft System in the treatment of type B aortic dissections.
Supported by the results of the DISSECTION trial, the new indication provides physicians with a minimally invasive alternative to open surgical repair and medical therapy for the condition.
“Acute type B aortic dissection is a potentially life-threatening condition that historically has been treated with either medical therapy or, when necessary, through invasive surgical techniques,” explained Joseph Bavaria, professor of Surgery and director of the Thoracic Aortic Surgery Program, University of Pennsylvania, USA, and a national principal investigator for DISSECTION.
DISSECTION results
Bavaria presented the results of the trial at the 2014 annual meeting of the Society for Thoracic Surgery. Twelve-month data from 50 patients evaluated in the trial demonstrate safety and efficacy of the Valiant Captivia System in the treatment of dissections, with excellent technical success.
Conducted at 16 US sites, the trial met its primary safety endpoint by achieving an 8% all-cause mortality rate at 30 days, which represents a three- to four-fold mortality improvement over open surgical repair. Additionally, 100% technical success and 100% coverage of the primary entry tear at implant were achieved in the trial.
Rodney White, chief of Vascular Surgery, Harbor-UCLA Medical Center, Torrrance, USA, added: “Data out to one year continue to show positive aortic remodelling of the stented segment, with a 100% increase in true lumen volume and no ruptures.”
Expansion in line of grafts
Medtronic recently expanded the size matrix of the Valiant Captivia Thoracic Stent Graft System with 11 new proximal FreeFlo tapered pieces, increasing configuration possibilities by 30% to address a wider range of patient anatomies. The line extension enables physicians to use the thoracic stent graft system in tapered aortas, which account for approximately 20% of all thoracic aortic aneurysm cases.The new pieces all taper by 4mm along their approximately 150mm length, and have proximal diameters that range from 26mm to 46mm. These additional system components received FDA approval, the CE mark and Health Canada approval in January, but their approved indications vary by geography.













