Vascular specialists at Carolinas HealthCare System in Charlotte, USA, and the Cleveland Clinic, Cleveland, USA, recently performed the initial implants of a novel stent graft system from Medtronic as part of a US Food and Drug Administration (FDA) initiative designed to encourage more early-stage clinical research on new medical devices in USA, according to a release. These implants were among the first to be performed under this FDA early feasibility pilot programme, which includes a total of nine medical devices from different companies.
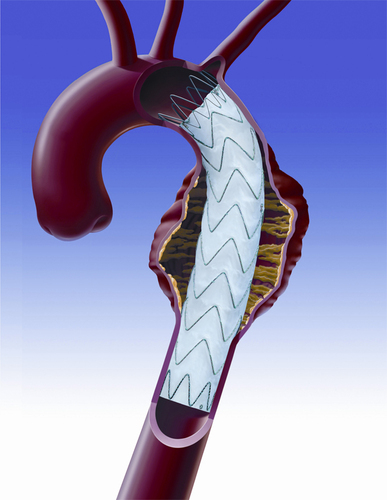
According to the release, the first device of its kind to undergo clinical evaluation anywhere in the world, Medtronic’s Valiant Mona LSA branch stent graft system is designed to enable the repair of thoracic aortic aneurysms encroaching on the left subclavian artery with an entirely endovascular approach. Its initial usage earlier this month marks a major step forward to develop standardised stent graft systems to treat aneurysms throughout the aorta when the involvement of a branch vessel requires the stent graft to allow perfusion of critical organs or tissue.
“A standardised stent graft system that addresses the anatomical variability in thoracic aortic aneurysms involving the left subclavian artery could make this repair technique even less invasive for a large number of patients,” said the study’s primary investigator, Eric Roselli, a cardiothoracic surgeon at the Cleveland Clinic, USA. “This trial is a first step toward developing more disease-specific and patient-specific devices to treat a very complex disease problem.”
Frank Arko, a vascular surgeon at Carolinas HealthCare System’s Sanger Heart and Vascular Institute, USA, and the site’s lead investigator, added: “This endovascular treatment for aortic aneurysms provides an important alternative to open-chest operations. By eliminating the need for invasive surgeries, we should be able to reduce certain complications and hopefully improve outcomes for patients facing a life-threatening illness.”
Approved by the FDA under an investigational device exemption, the clinical study of Medtronic’s Valiant Mona LSA branch stent graft system may enrol a total of seven patients at Carolinas HealthCare System and the Cleveland Clinic combined.
“This study will advance the development of future devices for the endovascular repair of aortic aneurysms that involve branch vessels,” said Tony Semedo, president and general manager of Medtronic’s Endovascular Therapies business. “It represents a gateway to stent grafts that could be used to treat aneurysmal disease across the aorta’s thoracic arch and ascending segment.”
The investigational device is based on the Valiant thoracic stent graft, which has been used to treat approximately 45,000 patients worldwide since 2005 when it received the CE mark. It features modifications to the standard device, including a branch cuff that accommodates the left subclavian artery branch graft.
Arko and Roselli are both paid consultants and speakers for Medtronic. Roselli serves on an unrelated Medtronic advisory board













