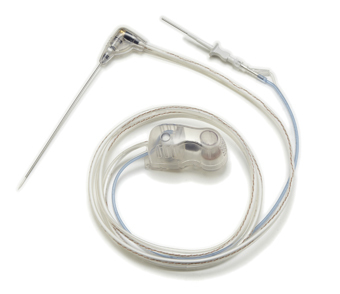
Microsulis Medical has received FDA clearance for its upgraded Accu2i pMTA applicator.
The initial version of the single, high-power, high-frequency 2.45GHz saline-cooled needle was cleared for use in the USA as part of the Acculis MTA system in 2010. Following two years of global distribution, and in response to customer feedback and growing product demand, Microsulis has updated the device’s design and manufacturing process. Improvements include a refined optically clear moulded handle and improved connection cartridge.
Stuart McIntyre, CEO of Microsulis Medical, said: “2.45GHz microwave ablation is becoming the new global standard for volume tissue coagulation, giving clinicians options for patients that were not possible with radiofrequency ablation technology.
According to the company, the new Accu2i pMTA applicator’s handle has undergone an ergonomic redesign, with ridged contact points and a more tactile feel. The optical grade clear applicator casing has been refined to allow the user to see the coolant flowing through the applicator. The coolant spike tubes have also been colour coded to provide a visual aid for ease of manufacture. The Accu2i pMTA cartridge, that connects the applicator to the Local Control Station (LCS), features also a more ergonomic design.
The Acculis MTA system was CE-marked in February 2010 and 510(k) cleared by the US Food and Drug Administration in August 2010. It is distributed globally, with the exception of the US, by AngioDynamics.
The FDA has cleared the Acculis MTA system for the coagulation of soft tissue.













