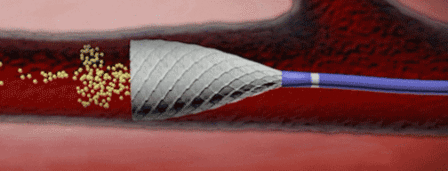
Surefire Medical has received CE mark approval for its Surefire Infusion System, a next generation device for chemo- and radioembolization procedures. The company has announced that the launch of this product will be effective immediately.
“Surefire Medical developed this ground-breaking technology to provide interventional radiologists with improved embolization certainty during their infusion procedures,” said Jim Chomas, CEO, Surefire Medical. “With the CE mark approval, we are able to introduce the Surefire Infusion System, which may offer substantial advantages over a standard microcatheter, throughout Europe.”
In a retrospective study of 29 patients, presented at the Society of Interventional Radiology (SIR) Annual Scientific Meeting 2012, infusion of therapeutic agent with the Surefire Infusion System showed no angiographic evidence of reflux and no clinical evidence of non-target embolization. CT imaging in chemoembolization patients suggested a pattern of increased microsphere tumor penetration and contrast retention.
Additionally, the Surefire High-Flow Microcatheter System recently received FDA 510(k) clearance. The Surefire Infusion System received FDA 510(k) clearance in June 2011.
As part of the international launch, Surefire Medical will participate in the upcoming annual meeting of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2012 in Lisbon, Portugal.












