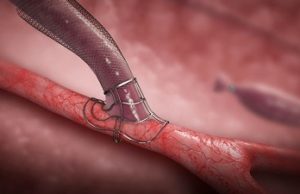
Jocelyn Hudson
Teleflex completes acquisition of Biotronik’s Vascular Intervention business
Teleflex today announced that is has completed the previously announced acquisition of substantially all of the Vascular Intervention business of Biotronik.
The company notes...
StentIt launches first-in-human trial of bioresorbable stent to treat below-the-knee CLTI
StentIt has announced the successful implantation of its Resorbable Fibrillated Scaffold (RFS). As part of the VITAL-IT 1 study, patients with chronic limb-threatening ischaemia...
New THRIVE data on Penumbra’s CAVT technology for acute limb ischaemia...
Recent THRIVE study data show that Penumbra’s computer-assisted vacuum thrombectomy (CAVT) technology not only has the potential to improve outcomes for lower extremity acute...
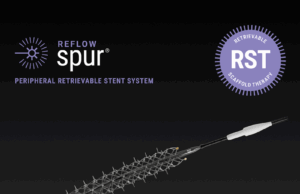
US FDA grants de novo clearance for Reflow Medical’s Spur peripheral...
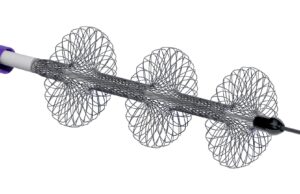
Reflow Medical recently announced that the US Food and Drug Administration (FDA) has granted de novo clearance for the company’s Spur peripheral retrievable stent...
Cost-effectiveness outcomes defy expectations in latest BEST-CLI data drop
A comment from Andrew Holden (Auckland City Hospital, Auckland, New Zealand) conveyed surprise at new cost-effectiveness data from the BEST-CLI trial that were shared...
Luminor drug-coated balloon launched in Japan
iVascular has announced that its Luminor drug-coated balloon (DCB) is now available in Japan.
“This approval from the MHLW is evidence of the quality,...
R3 Vascular announces first patient treated in ELITE-BTK pivotal trial
R3 Vascular recently announced that the first patient in its ELITE-BTK pivotal trial has been treated by Brian DeRubertis (New York-Presbyterian and Weill Cornell...
Cagent Vascular initiates patient enrolment in Serranator vs. plain balloon angioplasty...
Cagent Vascular has announced its first patient enrolment of the Serranator versus plain balloon angioplasty optical coherence tomography (OCT) study.
This prospective, randomised (2:1 treatment...
Microbot Medical shares results from ACCESS-PVI pivotal trial
Microbot Medical has shared that it presented for the first time the data from its ACCESS-PVI pivotal trial at the Society of Interventional Radiology...
Awaited first-release data and debates set to address critical challenges in...
“These crucial results will help to shape next steps in research, including ongoing randomised controlled trials comparing bioresorbable scaffolds to angioplasty, and eventually, to...
Real-world data show Cook’s Zilver PTX leads to lower rates of...
Cook Medical has announced that its Zilver PTX drug-eluting stent (DES) has lower rates of in-stent occlusions among patients with restenosis at three years than the Eluvia DES (Boston Scientific), according to...
Evident Vascular raises Series B financing to advance AI-powered IVUS platform...
Evident Vascular has announced the successful closing of its Series B financing with new investors Shangbay Capital and two undisclosed multinational strategics joining founding...
Imperative Care expands Symphony thrombectomy portfolio with new US FDA approval
Imperative Care today announced US Food and Drug Administration (FDA) 510(k) clearance of the 82cm version of its Symphony 16Fr catheter, the company’s latest...
Inari Medical, now part of Stryker, launches Artix thrombectomy system
Inari Medical, now part of Stryker, recently announced the launch of its Artix thrombectomy system. Purpose-built for the distinct needs of the peripheral arterial...
Stryker completes acquisition of Inari Medical
Stryker recently announced that it has completed the acquisition of Inari Medical. A press release notes that the addition of Inari brings an established...
Surmodics announces successful early clinical use of Pounce XL thrombectomy system
Surmodics has announced the successful early clinical use of its Pounce XL thrombectomy system. The device received US Food and Drug Administration (FDA) 510(k)...
Cagent Vascular launches Serranator SL-PRO for CLTI and pedal disease
Cagent Vascular has announced the launch of the Serranator SL-PRO percutaneous transluminal angioplasty (PTA) serration balloon catheter for chronic limb-threatening ischaemia (CLTI) and pedal...
Data and device tweaks herald ‘practice-changing’ future for bioabsorbable scaffolds
Pending the results of several trials and “iterative changes” to device design, bioabsorbable scaffolds are set to change the treatment paradigm for lower extremity...
AngioDynamics initiates AMBITION BTK RCT and registry to advance treatment for...
AngioDynamics today announced the initiation of AMBITION BTK, a randomised study of the Auryon atherectomy system in the treatment of below-the-knee (BTK) chronic limb-threatening...
Cagent Vascular initiates the Serranator POINT FORCE observational registry
Cagent Vascular has announced the start of the POINT FORCE registry, a postmarket clinical follow-up study of the Serranator percutaneous transluminal angioplasty (PTA) serration...
AVS completes US$36 million Series B financing ahead of IVL US...
Amplitude Vascular Systems (AVS) recently announced that it has completed a Series B round of financing of US$36 million. The funding will support the...
Drug-eluting technologies should be ‘de facto standard of care’ for PAD,...
New data from a large, real-world study support the use of drug-eluting devices to reduce amputations, readmissions, and healthcare costs in the treatment of...
Bentley launches BeFlow iliac covered stent system at LINC 2025
Bentley today announced the market launch of its BeFlow iliac covered stent system at the Leipzig Interventional Course (LINC 2025; 28–30 January, Leipzig, Germany)....
Concept Medical’s SirPAD randomised trial completes patient enrolment
Concept Medical has announced the successful completion of patient enrolment in the SirPAD trial, with over 1,250 patients now enrolled.
A press release notes that...
First patient enrolled in Concept Medical’s MAGICAL BTK IDE trial
Concept Medical has successfully enrolled the first patient in the MAGICAL BTK US investigational device exemption (IDE) pivotal trial, a press release reveals.
Following the...
R3 Vascular secures WCG IRB approval and CMS category B Medicare...
R3 Vascular today announced that it has received WCG Institutional Review Board (IRB) approval for the ELITE-BTK pivotal trial of its Magnitude drug-eluting next-generation...
Study finds adverse outcomes after decreased use of paclitaxel-coated devices
A recent analysis of over 270,000 Medicare fee-for-service beneficiaries has found an increase in adverse outcomes and death after a US Food and Drug...
Stryker announces definitive agreement to acquire Inari Medical
Stryker has announced a definitive agreement to acquire all of the issued and outstanding shares of common stock of Inari Medical for US$80 per...
Biotronik enrols first patients in BIO-OSCAR FIRST trial
Biotronik announced this week that it has enrolled the first patients in its BIO-OSCAR FIRST trial, a study designed to confirm the safety and...
Kathleen Van Vlierberghe appointed vice president of Peripheral Interventions for Boston...
Boston Scientific today announced the appointment of Kathleen Van Vlierberghe as the new vice president for the Peripheral Interventions division in Europe, Middle East and...
Sensome announces data from two studies showing clot-sensing guidewire successfully identifies...
Sensome today announced positive results from two studies of its Clotild smart guidewire system demonstrating its ability to successfully identify fresh clot—thrombus rich in...
BIO-OSCAR SOC trial examines standard of care in PAD treatment
Biotronik today announced that it has concluded the BIO-OSCAR SOC study evaluating the baseline against which to measure the Oscar multifunctional catheter in treating complex...
Endologix announces 36-month results of DETOUR2 study
Endologix has announced the final 36-month results from the DETOUR2 study—a prospective, single-arm, international, multicentre clinical evaluation of the novel Detour system for fully...
Shockwave Javelin peripheral IVL catheter meets prespecified efficacy and safety performance...
Shockwave Medical, part of Johnson & Johnson MedTech, has announced the first clinical outcomes associated with the Shockwave Javelin peripheral intravascular lithotripsy (IVL) catheter,...
Bolt Medical announces completion of RESTORE ATK and RESTORE BTK pivotal...
Bolt Medical has announced the completion and results of the RESTORE ATK and RESTORE BTK pivotal clinical trials investigating the company's Bolt intravascular lithotripsy...
Two-year LIFE-BTK data show sustained benefits of drug-eluting resorbable scaffold for...
Presented today, late-breaking data from the second year of the LIFE-BTK clinical trial demonstrate the long-term effectiveness of the US Food and Drug Administration...
US FDA grants R3 Vascular IDE approval for ELITE-BTK pivotal trial...
R3 Vascular today announced that the US Food and Drug Administration (FDA) has granted investigational device exemption (IDE) approval to initiate its ELITE-BTK pivotal...
TCT 2024: Drug-eluting resorbable scaffold proves cost effective at one year...
A retrospective economic analysis of the LIFE-BTK trial has demonstrated the one-year cost-effectiveness of an everolimus-eluting resorbable scaffold over angioplasty for the treatment of...
CMS grants transitional pass-through payment for Medtronic’s Symplicity Spyral and Recor’s...
The Centers for Medicare and Medicaid Services (CMS) has granted transitional pass-through (TPT) payment for Medtronic's Symplicity Spyral renal denervation (RDN) catheter and Recor...
Surmodics announces early results from PROWL registry of Pounce thrombectomy system
Surmodics has announced that early results of a subset of 60 real-world acute, subacute, and chronic limb ischaemia patients from its PROWL registry study...
Vesalio announces clinical study initiative for recently launched pVasc thrombectomy system
Vesalio has announced the initiation of a prospective, single-arm, multicentre study supporting the recently launched pVasc thrombectomy system for non-surgically removing peripheral occlusions. This...
Novel RCT framework set to improve design and reporting of endovascular...
Pilot testing has shown that a new framework—dubbed Endo-STAR—can be used to describe and standardise endovascular interventions for peripheral arterial disease (PAD) within a...
AVS enrols first patient in US pivotal intravascular lithotripsy study
Amplitude Vascular Systems (AVS) recently announced that it has enrolled the first patient in its US pivotal trial for pulsatile intravascular lithotripsy (IVL) therapy....
Contego Medical’s Neuroguard IEP system receives US FDA approval for carotid...
Contego Medical today announced the US Food and Drug Administration's (FDA) premarket approval (PMA) of the Neuroguard IEP system for carotid revascularisation.
The company shares...
Philips announces US FDA approval for enhanced LumiGuide guidewire, marks 1000th...
Royal Philips today announced the introduction of the 160cm US Food and Drug Administration (FDA)-approved version of its LumiGuide endovascular navigation wire. This enhanced...
Shockwave Medical expands US peripheral IVL portfolio with enhanced catheter
Shockwave Medical, part of Johnson & Johnson MedTech, has announced the full US launch of its Shockwave E8 peripheral intravascular lithotripsy (IVL) catheter, following...
Penumbra receives CE mark for Lightning Flash 2.0 and Lightning Bolt...
Penumbra today announced it has secured CE mark in Europe for two computer-assisted vacuum thrombectomy (CAVT) technologies—Lightning Flash 2.0 and Lightning Bolt 7.
“Based on...
New vessel preparation and drug delivery data spotlighted at LINC 2024
Sabine Steiner (University of Leipzig, Leipzig, Germany) took to the podium at this year’s Leipzig Interventional Course (LINC 2024; 28–31 May, Leipzig, Germany) to...
AngioDynamics announces CE mark approval in Europe for the Auryon system
AngioDynamics today announced European CE mark approval of the Auryon atherectomy system, designed for the treatment of peripheral arterial disease (PAD), including chronic limb-threatening...
Study: Social support associated with better PAD health outcomes
Patients with peripheral arterial disease (PAD) reporting lower levels of social support experience worse health outcomes, a new Yale-led study finds.
Social support is thought...
New ESC guidelines combine peripheral arterial and aortic diseases for first...
The European Society of Cardiology (ESC) has today published its 2024 guidelines for the management of peripheral arterial and aortic diseases (PAAD), evaluating these...
SCOREPAD seeks to address “alarmingly high” mortality in CLTI patients with...
A new research letter underscores the need to improve long-term survival following lower extremity revascularisation for chronic limb-threatening ischaemia (CLTI), setting out a randomised...
Claudication: Long-term Medicare study highlights “critical” need for value-based care
A newly published US Medicare cohort study illuminates trends and factors associated with peripheral vascular interventions (PVIs)—including a sharp rise in the use of...
Transit Scientific’s XO Cath microcatheter used in radial access UAE procedure...
Transit Scientific recently announced a milestone achievement with the successful utilisation of XO Cath, its embolic delivery microcatheter, via transradial access without a support...
First results of SOL Japan highlight “extraordinary performance” of Luminor DCB...
iVascular has announced that "outstanding" results from the SOL Japan study were recently presented at the Japan Endovascular Treatment (JET) conference (14–16 June, Fukuoka,...
Endovascular CFA treatment associated with increased rate of long-term CFA-specific reintervention,...
A recent study suggests that endovascular treatment of the common femoral artery (CFA) is associated with an increased rate of long-term CFA-specific reintervention, regardless...
AVS receives IDE approval from US FDA for pivotal intravascular lithotripsy...
Amplitude Vascular Systems (AVS) announced today that it has received an investigational device exemption (IDE) from the US Food and Drug Administration (FDA) to...
LINC 2024: C-GUARDIANS data represent lowest event rates in published trials...
New data from the C-GUARDIANS pivotal investigational device exemption (IDE) trial support consideration of carotid artery stenting (CAS) with the CGuard embolic prevention stent...
Johnson & Johnson completes acquisition of Shockwave Medical
Johnson & Johnson today announced it has completed its acquisition of Shockwave Medical. Shockwave is now part of Johnson & Johnson and will operate...
NICE publishes a guidance supporting the use of selective internal radiation...
Terumo Europe recently shared that a positive guidance has been published by the National Institute for Health and Care Excellence (NICE) stating that selective...
PAD guideline update from ACC, AHA and others centres multispecialty care
New multisociety clinical practice guidelines for the management of lower extremity peripheral arterial disease (PAD) have been published online in the Journal of the...
Ultrafast contrast-enhanced ultrasound for PAD patients shows promise in early feasibility...
Majorie van Helvert (University of Twente, Enschede, The Netherlands) and Michel Reijnen (Rijnstate Hospital, Arnhem, The Netherlands) are currently conducting research on ultrafast contrast-enhanced...
FastWave Medical granted fifth utility patent reflecting continuous expansion of IVL...
FastWave Medical has been issued its fifth utility patent by the United States Patent and Trademark Office (USPTO), a press release reveals.
"FastWave's IVL ...
Studies highlight need for tailored treatment options for women with peripheral...
New clinical results highlight the need for inclusive approaches and comprehensive examinations of treatment options for peripheral arterial disease (PAD), including endovascular therapy and...
Reflow Medical completes enrolment in the DEEPER REVEAL clinical trial
Reflow Medical has announced completion of enrolment in the DEEPER REVEAL clinical trial to evaluate the Reflow Spur stent.
The company notes in a...
Expanse Ice aspiration system receives US FDA clearance for vessels of...
Expanse Ice recently announced that its Ice aspiration system has received 510(k) clearance from the US Food and Drug Administration (FDA).
A press release notes...
Provisio Medical announces US FDA clearance of the Provisio SLT IVUS...
Provisio Medical has announced US Food and Drug Administration (FDA) 510(k) clearance of the Provisio sonic lumen tomography (SLT) intravascular ultrasound (IVUS) system.
According...
Rotarex™S: A 25-year legacy continues
This advertorial, sponsored by BD, is only available in selected countries and geographies.
This year marks a significant milestone as BD celebrates 25 years...
New data show Penumbra’s Indigo aspiration system used in a single...
During the Society of Interventional Radiology (SIR) 2024 annual meeting (23–28 March, Salt Lake City, USA), newly presented data from a subgroup analysis of...
Endovascular showdown: CX 2024 sets the stage for BASIL-3 first-time data...
At the Charing Cross (CX) Symposium 2024 (23–25 April, London, UK), Andrew Bradbury (University of Birmingham, Birmingham, UK) and the BASIL-3 team of triallists...
Hans-Henning Eckstein: 1955–2024
Hans-Henning Eckstein, the vascular surgeon who played a leading role in the SPACE and SPACE 2 randomised controlled trials on the treatment of carotid...
US FDA Breakthrough Device designation granted for Biotronik’s Freesolve BTK resorbable...
Biotronik has been granted Breakthrough Device designation (BDD) from the US Food and Drug Administration (FDA) for the Freesolve below-the-knee (BTK) resorbable magnesium scaffold...
Endologix initiates postmarket study of the Detour system
Endologix has announced the initiation of the Percutaneous transmural arterial bypass (PTAB)1 postmarket study. This study marks the beginning of a comprehensive postmarket study...
Cagent Vascular raises US$30 million series C financing
Cagent Vascular has announced a series C financing close in excess of US$30 million. US Venture Partners (USVP) led the round. Participation included new...
IsomAb announces close of £7.5 million financing to accelerate lead candidate...
IsomAb Ltd, a UK-based biotechnology company, has announced the closing of a £7.5 million (approximately US$9.4 million) seed financing round, led by Broadview Ventures, with...
Efemoral Medical granted Breakthrough Device designation
Efemoral Medical today announced that the US Food and Drug Administration (FDA) has granted its novel Efemoral vascular scaffold system (EVSS) Breakthrough Device status...
Surmodics announces successful early clinical use of Pounce LP thrombectomy system
Surmodics has announced successful early clinical use of the company’s Pounce LP (low-profile) thrombectomy system. The Pounce LP system, which received US Food and Drug...
AngioDynamics announces US FDA 510(k) clearance of Auryon XL radial access...
AngioDynamics today announced that the US Food and Drug Administration (FDA) has cleared the Auryon XL catheter, a 225cm radial access catheter, for use with...
Surmodics announces successful early clinical use of Pounce LP thrombectomy system
Surmodics has announced successful early clinical use of the company’s Pounce LP (low-profile) thrombectomy system. The Pounce LP system, which received US Food and Drug...
Reflow Medical receives CE mark for Bare Temporary Spur stent system
Reflow Medical recently announced it has received CE mark certification in the European Union for the Bare Temporary Spur stent system. The device is...
First patients enrolled in Gore’s VBX FORWARD clinical study
Gore has announced that the first patients have been enrolled in the Gore VBX FORWARD clinical study, a global prospective, multicentre, randomised controlled trial...
US Centers for Medicare & Medicaid Services grants Transitional Pass-Through payment...
Endologix recently announced that the US Centers for Medicare & Medicaid Services (CMS) has granted a Transitional Pass-Through (TPT) payment for the Detour system,...
Inari Medical announces first patient enrolment in PEERLESS II randomised controlled...
Inari Medical has announced the first patient enrolment in PEERLESS II. This prospective, global, multicentre randomised controlled trial (RCT) compares the outcomes of intermediate-risk...
First patient enrolled in STORM-PE RCT evaluating Penumbra’s Lightning Flash for...
Penumbra today announced that the first patient has been enrolled in STORM-PE, a prospective, multicentre, randomised controlled trial (RCT) evaluating anticoagulation alone versus anticoagulation...
Two-year BIOPACT randomised controlled trial analysis demonstrates “persistent excellence” for low-profile...
Biotronik has announced the presentation of two-year results from the investigator-initiated BIOPACT randomised controlled trial (RCT) by principal investigator Koen Deloose (AZ Sint-Blasius Hospital,...
Cordis announces enrolment completion of the RADIANCY clinical study in Europe
Cordis has announced the completion of patient enrolment in the RADIANCY premarket clinical study. The prospective, multicentre, single-arm study is designed to evaluate the...
Two-year SWING data “continue to show promise” for sirolimus DCB in...
Two-year data from the SWING trial, a first-in-human study of the safety and performance of the Sundance sirolimus drug-coated balloon (DCB; Surmodics), have been...
Duo venous stent system shows 90.2% primary patency at 12 months,...
The Duo venous stent system (Vesper Medical/Philips) showed a 98.7% freedom from major adverse events (MAEs) at 30 days and 90.2% primary patency at...
Endologix announces results of pooled analysis of DETOUR1 and DETOUR2 Studies...
Endologix has announced results from a pooled analysis of the DETOUR1 and DETOUR2 studies evaluating percutaneous transmural arterial bypass (PTAB) with the Detour system.
A...
LimFlow announces agreement to be acquired by Inari Medical
LimFlow announced today that it has entered into a definitive agreement to be acquired by Inari Medical.
A press release details that, under the terms...
One-year CLOUT data demonstrate low rate of PTS following treatment with...
David Dexter (Sentara Vascular Specialists, Norfolk, USA) shared one-year data from the CLOUT registry this week at The VEINS 2023 (28–30 October, Las Vegas,...
REAL-PE demonstrates statistically significant lower major bleeding rates with Ekos system...
Data from the REAL-PE study were presented this week at TCT 2023 (23–26 October, San Francisco, USA) demonstrating that patients treated for pulmonary embolism...
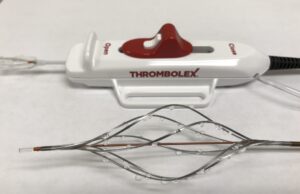
Thrombolex announces new insights from the RESCUE trial with the Bashir...
Thrombolex has announced never-before-reported major reductions in obstruction in all of the segmental pulmonary arteries (PA), based on independent core lab data analysis of...
TCT 2023: LIFE-BTK breathes life into drug-eluting resorbable scaffolds in breakthrough...
Results of the LIFE-BTK randomised controlled trial have just been presented at TCT 2023 (23–26 October, San Francisco, USA). The data show that, in...
Cardio Flow announces US FDA 510(k) clearance for its FreedomFlow orbital...
Cardio Flow recently announced it has received US Food and Drug Administration (FDA) 510(k) clearance for the company’s FreedomFlow orbital atherectomy peripheral platform.
The company...
Obituary: Roger M Greenhalgh 6th February 1941 – 6th October 2023
Roger Malcolm Greenhalgh, the surgeon internationally renowned for his unparalleled contribution to vascular education, training and research, died peacefully on 6th October, aged 82....
Multi-society guidelines on varicose vein management published
The Society for Vascular Surgery (SVS), American Venous Forum (AVF), and American Vein and Lymphatic Society (AVLS) have released the second and final part...
FDA update: Paclitaxel-coated devices for PAD cleared of excess mortality risk,...
This advertorial is sponsored by Boston Scientific.
Data on hundreds of thousands of patients who have received lower limb endovascular treatment provide confidence in paclitaxel...
Humacyte completes enrolment in Phase II/III trial of Human Acellular Vessel...
Humacyte today announced completion of enrolment in its Phase II/III vascular trauma trial (V005) that is expected to support a Biologics License Application (BLA)...
Biotronik announces one-year subgroup results from BIOPACT RCT
Biotronik has announced one-year subgroup results from the investigator-initiated BIOPACT randomised controlled trial (RCT), which were presented by principal investigator Koen Deloose (AZ Sint-Blasius,...
Boston Scientific announces position on FDA update about use of paclitaxel-coated...
Following yesterday's news that the US Food and Drug Administration (FDA) has changed its stance on the use of paclitaxel-coated devices to treat peripheral...
RECOIL study: Serranator demonstrates 49% less recoil than plain balloon angioplasty...
Cagent Vascular has announced the results of its below-the-knee (BTK) RECOIL study. This core lab-adjudicated Recoil analysis— the first of its kind, according to...
Surmodics receives FDA approval for the SurVeil drug-coated balloon
Surmodics has announced the receipt of US Food and Drug Administration (FDA) approval for the SurVeil drug-coated balloon (DCB).
A company press release notes that...
Inari Medical announces commercial launch of RevCore and Triever16 Curve for...
Inari Medical today announced the launch of two new purpose-built products, the RevCore thrombectomy catheter, and the Triever16 Curve catheter.
According to a company press...
Biotronik launches Oscar multifunctional peripheral catheter at LINC 2023
Biotronik today announced the limited release of its Oscar (One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter and start of promotional activities at this...
“Excellent” 12-month results from SELUTION SFA trial presented
Twelve-month results from the SELUTION SFA trial have been presented for the first time at the Japan Endovascular Treatment (JET) Conference (26–28 May, Tokyo,...
MagicTouch sirolimus-coated balloon granted IDE approval for treatment of the SFA
On 24 May, the US Food and Drug Administration (FDA) granted an investigational device exemption (IDE) approval for Concept Medical's MagicTouch percutaneous transluminal angioplasty (PTA)...
Urban residents have smaller risk of mortality from chronic limb-threatening ischaemia
A new study using data from millions of patients hospitalised across the USA has determined whether population density and associated urban versus suburban versus...
Insights from largest chronic limb-threatening ischaemia study to inform quality of...
Data from a cohort of one million patients with chronic limb-threatening ischaemia (CLTI) were recently presented as late-breaking clinical research at the Society for Cardiovascular...
Mechanical thrombectomy: ClotTriever offers “extended window” for DVT treatment
This advertorial, sponsored by Inari Medical, is only available in selected countries and geographies.
During a recent webinar hosted by Inari Medical, a multidisciplinary group...
New analysis of chronic limb-threatening ischaemia patients places BEST-CLI trial into...
A new analysis of chronic limb-threatening ischaemia (CLTI) treatment outcomes was presented today as late-breaking clinical research at the Society for Cardiovascular Angiography &...
Bentley launches its first product in the USA
Bentley has announced the US launch of its BeBack crossing catheter, which is designed for the treatment of heavily calcified lesions. This is the...
New “biopsychosocial” roadmap set out to address PAD treatment and mental...
A recent proposal has called for “whole-person, multidisciplinary interventions” after an interrogation of the interplay between lower extremity peripheral arterial disease (PAD) and mental...
Akura Medical announces successful first-in-human use of its mechanical thrombectomy platform
Akura Medical announced today it has initiated its first-in-human clinical study of the Akura mechanical thrombectomy platform. A press release notes that the Akura...
Five-year results of the LEOPARD trial published in the Journal of...
Endologix has announced the online publication of the final five-year results of the LEOPARD trial in the Journal of Vascular Surgery (JVS). The study's...
Real-world data consistent with RCTs in highlighting the efficacy of drug-eluting...
This advertorial is sponsored by Boston Scientific.
During a recent satellite symposium, which took place at the 2022 Cardiovascular Interventional Radiological Society of Europe (CIRSE)...
BASIL-2 points towards endovascular-first revascularisation strategy in CLTI patients
A question from Manj Gohel (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) on what the Charing Cross (CX) audience should take back to...
BASIL-2 points towards endovascular-first revascularisation strategy in CLTI patients
A question from Manj Gohel (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) on what the Charing Cross (CX) audience should take back to...
Surmodics announces successful first patient use of Sublime radial access microcatheter...
Surmodics has announced that Ankur Lodha (Cardiovascular Institute of the South, Lafayette, USA) and Pradeep Nair (Cardiovascular Institute of the South, Houma, USA) were...
AVS’s pulsatile IVL technology attracts an additional US$8.8 million to close...
AVS, an early-stage medical device company focused on safely and effectively treating severely calcified arterial disease with its pulsatile intravascular lithotripsy (PIVL) therapy, has...
Results from PROMISE II pivotal trial published, reinforcing “transformational value” of...
Results from the PROMISE II pivotal trial investigating transcatheter arterialisation of the deep veins using the LimFlow system in so-called no-option chronic limb-threatening ischemia...
CX 2023 highlight: The BASIL-2 trial
Data and discussion on revascularisation treatment strategies for patients with chronic limb-threatening ischaemia (CLTI) will take centre stage at the CX 2023 Consensus update,...
Algorithm predicts females have higher risk for kidney damage after aneurysm...
When receiving treatment for abdominal aortic aneurysm (AAA), female patients have a higher risk for kidney damage after endovascular repair, a Michigan Medicine study...
Shockwave Medical announces US launch of new peripheral IVL catheter
Shockwave Medical today announced the full US commercial availability of the Shockwave L6 peripheral intravascular lithotripsy (IVL) catheter following clearance by the US Food...
New VOYAGER PAD analysis confirms consistent benefit of low-dose rivaroxaban plus...
Data from a new prespecified analysis of the phase III VOYAGER PAD clinical trial show that low-dose rivaroxaban plus aspirin resulted in a 33%...
Viz.ai to expedite patient enrolment in NIH-funded PE-TRACT clinical trial
Viz.ai has announced it will use its Viz Recruit platform to optimise patient enrolment for the National Institutes of Health (NIH)-funded Pulmonary embolism—thrombus removal...
Results on ReCor Medical’s Paradise ultrasound renal denervation system published in...
ReCor Medical and its parent company, Otsuka Medical Devices, recently announced that primary endpoint results from the RADIANCE II pivotal trial were published in...
Biotronik launches Oscar multifunctional peripheral catheter
Biotronik has announced the US Food and Drug Administration (FDA) 510(k) clearance and CE mark of its Oscar (One Solution: Cross. Adjust. Restore) multifunctional...
Stenting of the SFA for intermittent claudication yields quality-of-life benefits out...
In a recent study of patients with intermittent claudication (IC) caused by isolated superficial femoral artery (SFA) lesions, researchers found that primary stenting conferred...
Northeast Scientific awarded 510(k) clearance to reprocess the Eagle Eye Platinum...
Northeast Scientific announced this week it has received US Food and Drug Administration (FDA) 510(k) clearance for reprocessing the intravascular ultrasound (IVUS) Eagle Eye...
The BeBack crossing catheter: A “game-changer” in endovascular PAD practice
This advertorial is sponsored by Bentley.
Crossing chronic total occlusion (CTO) lesions are challenging procedures. The BeBack crossing catheter—Bentley’s first product to be available in...
PRELUDE-BTK subanalysis “suggests advantage” for serration angioplasty
Cagent Vascular has announced the results of a comparative subanalysis of the PRELUDE-below-the-knee (BTK) study versus plain balloon angioplasty.
The study was led by Marianne...
Surmodics provides regulatory update related to its FDA premarket approval application...
Surmodics recently announced it has received a letter from the US Food and Drug Administration (FDA) related to its premarket approval (PMA) application for...
ESVS AAA guidelines alert recommends “enhanced surveillance” in patients treated with...
In a newly released “focused update” to their 2019 recommendations, the European Society for Vascular Surgery (ESVS) abdominal aortic aneurysm (AAA) guidelines writing committee...
Viz.ai launches AI-powered Viz Vascular Suite
Viz.ai has announced the launch of Viz Vascular Suite—artificial intelligence (AI)-powered software enabling vascular care teams to automatically detect and triage care for suspected...
Cardiovascular Systems enrols first patient in Japan for KAIZEN clinical study
Cardiovascular Systems Inc (CSI) recently announced initiation of the KAIZEN clinical study of its Diamondback 360 peripheral orbital atherectomy system (OAS) for the treatment...
Penumbra launches Lightning Flash mechanical thrombectomy system
Today, Penumbra announced the US Food and Drug Administration (FDA) clearance and launch of its Lightning Flash mechanical thrombectomy system.
"Lightning Flash features Penumbra's novel...
SWING trial 12-month data: Novel sirolimus DCB shows “great promise” in...
The Sundance (Surmodics) sirolimus drug-coated balloon (DCB) has an “excellent” safety profile in a “challenging, real-world, predominantly CLTI population,” and has a primary...
Mechanical thrombectomy for DVT: Randomised data needed to boost growing evidence...
Two datasets presented during the late-breaking clinical trials session at The VEINS (Venous Endovascular Interventional Strategies) 2022 (30–31 October, Las Vegas, USA)—the latest results...
“Significant” increase in atherectomy use in the USA largely driven by...
In the USA, atherectomy use in peripheral vascular interventions (PVIs) “more than doubled” from 2010 to 2019, with office-based procedures a “major driver” of...
Cordis announces acquisition of MedAlliance
Cordis today announced its acquisition, subject to customary closing conditions including regulatory approvals, of MedAlliance.
A press release notes that the agreement includes an...
Cardiovascular Systems announces full market release of the 2.00 Max Crown...
Cardiovascular Systems Inc (CSI) has announced the full market release of the 2.00 Max Crown for peripheral orbital atherectomy systems (2.00 Max Crown).
CSI notes...
ABRE clinical study 36-month data show sustained effectiveness of Abre venous...
Medtronic has announced the 36-month final results from the ABRE clinical study. The purpose of the ABRE clinical study was to evaluate the safety...
Six-month SWING trial data show promise for Sundance DCB
Six-month data from the Surmodics SWING first-in-human (FIH) study of the company’s Sundance sirolimus drug-coated balloon (DCB) were shared at the 2022 Amputation Prevention...
Preliminary MOTIV BTK outcomes positive for bioresorbable scaffold use in below-the-knee...
Thomas Rand (Klinik Florisdorf, Vienna, Austria) recently presented on the preliminary results to 12 months of the Motiv bioresorbable scaffold (Reva Medical) postmarket trial...
FLASH results demonstrate “excellent safety profile” of the FlowTriever system in...
Results of the FLASH registry demonstrate the “excellent safety profile” of the FlowTriever system (Inari Medical) in 800 “real-world” patients. This is according to...
Terumo announces study results underscoring safety and efficacy of radial to...
Terumo Medical Corporation has announced late-breaking data showing the safety and efficacy of radial to peripheral (R2P) interventions with the R2P Misago self-expanding peripheral...
Bentley acquires GoBack catheter from Upstream Peripheral Medical Technologies
Bentley today announced that it has acquired the rights of the GoBack catheter from Upstream Peripheral Technologies.
“The acquisition of the GoBack catheter marks the...
ABK Biomedical announces FDA 510(k) clearance of Easi-Vue embolic microspheres for...
ABK Biomedical has announced US Food and Drug Administration (FDA) 510(k) clearance of Easi-Vue embolic microspheres for the treatment of patients suffering from arteriovenous...
ClotTriever deemed a “game-changer” in deep vein thrombosis treatment
This advertorial, sponsored by Inari Medical, is only available in selected countries and geographies.
“I am finally very confident I have a device that...
Multidisciplinary community unites at inaugural patient-focused ESKD Summit
This advertorial, sponsored by BD, is only available in selected countries and geographies.
“The Changing Face of a Dialysis Patient” was the central theme...
Inari Medical announces randomised controlled trial evaluating clinical outcomes of the...
Inari Medical has announced planned enrolment of the DEFIANCE randomised controlled trial (RCT), which is designed to compare the clinical outcomes of patients with...
Vascular Therapies initiates enrolment in ACCESS 2 clinical trial
Vascular Therapies has announced that the first patient in the ACCESS 2 clinical trial was enrolled by Nikhil Kansal at Harbor-UCLA Medical Center in Torrance, USA.
The...
Study is first to elucidate lower limb amputation epidemiology in a...
A study recently published in the World Journal of Surgery claims to be the first to provide comprehensive population-level data on the epidemiology of...
First US patient enrolled in Selution SLR IDE peripheral study
The first US patient has been enrolled in the US Food and Drug Administration (FDA) SELUTION4BTK (below-the-knee) clinical trial evaluating Selution SLR, MedAlliance's novel...
BD launches first-in-human trial of a peripheral sirolimus drug-coated balloon
BD has announced the start of enrolment in a first-in-human trial of a peripheral sirolimus drug-coated balloon (DCB).
The PREVISION trial is a prospective, multicentre,...
Recurrent varicose veins: Multiple tools available to achieve excellent outcomes
Ramona Gupta (Northwestern University, Chicago, USA) addresses the issue of recurrent varicose veins, highlighting in particular the “multiple tools” now at physicians’ disposal to...
Vascular experts establish appropriate use of IVUS in peripheral interventions
Royal Philips today announced an important milestone in the evolving standard of care for treating patients with peripheral vascular disease: the establishment of the...
AngioDynamics announces FDA clearance of expanded indications for Auryon atherectomy system
AngioDynamics recently announced that it has received US Food and Drug Administration (FDA) 510(k) clearance of an expanded indication for the Auryon atherectomy system...
Shape Memory Medical celebrates treatment of 1,500th patient
Shape Memory Medical announced today that its 1,500th patient has been treated, marking a significant milestone for the company’s portfolio of shape memory polymer...
First patients enrolled in LIMES randomised trial of Magic Touch sirolimus-coated...
Concept Medical has announced the initiation of the LIMES trial, which has enrolled 15 patients to evaluate the safety and efficacy of the company's...
Transit Scientific announces XO Cross success in CLTI pedal artery access
Transit Scientific has reported multiple successful peripheral vascular procedures with the XO Cross catheter platform utilising pedal retrograde access.
The non-tapered 2Fr XO Cross...
Biotronik’s Pulsar-18 T3 peripheral self-expanding stent system receives FDA approval
Biotronik recently announced that it has received US Food and Drug Administration (FDA) approval of its Pulsar-18 T3 peripheral self-expanding stent system. Full US...
Cordis announces start of enrolment in RADIANCY clinical study in Europe
Cordis has announced the start of the RADIANCY premarket clinical study in Europe.
A press release details that RADIANCY is a prospective, multicentre, single-arm...
SoniVie announces successful procedure with Tivus system on first patient enrolled...
SoniVie today announced that on 30 May this year the first patient was treated with its renal artery denervation Tivus therapeutic intravascular ultrasound technology, as...
New data on Biotronik’s Passeo-18 Lux DCB presented at LINC 2022
Biotronik announced the presentation of two studies on the performance of its drug-coated balloon (DCB) catheter Passeo-18 Lux at the Leipzig Interventional Course (LINC)...
Ra Medical Systems receives FDA 510(k) clearance for the Dabra 2.0...
Ra Medical Systems has announced receipt of US Food and Drug Administration (FDA) 510(k) clearance for the company’s Dabra 2.0 catheter as part of...
SoniVie receives FDA IDE approval for pilot study to treat hypertension...
SoniVie recently announced that on 5 May 2022 the US Food and Drug Administration (FDA) granted investigational device exemption (IDE) approval for its REDUCED1...
Cordis announces successful first-in-human use of the Radianz radial peripheral system
Cordis recently announced the first-in-human use of the Radianz radial peripheral system by Jihad Mustapha at Advanced Cardiac & Vascular Centers (ACV) Grand Rapids,...
Philips announces positive three-year clinical research results from its TOBA II...
Royal Philips today announced the latest results from the Tack optimised balloon angioplasty (TOBA) II below-the-knee (BTK) clinical trial, demonstrating that the Philips endovascular...
Cardio Flow announces FDA clearance for FreedomFlow peripheral guidewire
Cardio Flow has announced it recently received US Food and Drug Administration (FDA) clearance for the company’s FreedomFlow peripheral guidewire.
According to a company press...
VentureMed completes enrolment of Flex Vessel Prep system randomised controlled trial...
VentureMed Group recently announced that it has completed enrolment of a randomised controlled trial (RCT) titled 'Flex Vessel Prep prior to PTA for the...
Shockwave Medical and Genesis MedTech obtain regulatory approval in China for...
Shockwave Medical and Genesis MedTech Group announced today that they have successfully obtained approval from China’s National Medical Products Administration (NMPA) to market and...
New long-term data of paclitaxel devices continue to show no increased...
New long-term data from the SAFE-PAD (Safety assessment of femoropopliteal endovascular treatment with paclitaxel-coated devices) study were presented today as late-breaking clinical research at...
Shockwave IVL maintains superiority to angioplasty in calcified peripheral disease at...
Shockwave Medical announced today that long-term data from the Disrupt PAD III trial found that superior vessel preparation with intravascular lithotripsy (IVL) led to...
RANZCR releases statement on iodinated contrast media shortage
The Royal Australian and New Zealand College of Radiologists (RANZCR) has issued a statement addressing the Australian and global shortage of iodinated contrast media.
The...
Vivasure Medical announces Series D financing to advance portfolio of PerQseal...
Vivasure Medical has announced the closing of the first tranche of €22 million (US$23 million) as part of its Series D financing round that...
Pounce thrombectomy system first-in-human data show 100% technical success in early...
Surmodics has announced that its Pounce thrombectomy system achieved 100% technical success in 20 first-in-human (FIH) procedures. The FIH data were presented by Gary...
Penumbra announces the European launch of the Indigo system with Lightning...
Penumbra has announced that its Indigo aspiration system with Lightning 7 and Lightning 12 have secured CE mark and are now commercially available in...
Study finds increased risk of serious blood clots up to six...
A study from Sweden published by The BMJ recently finds an increased risk of deep vein thrombosis (DVT) up to three months after COVID-19 infection,...
LimFlow raises US$40 millions in Series D financing
LimFlow recently announced it has closed a US$40 million (€36 million) oversubscribed Series D financing round.
A press release reports that new investors Longitude...
Cordis makes strategic investment in E2, a developer of next-generation thrombectomy...
Cordis has announced a strategic investment venture that will expand the scope of the global cardiovascular technology company into the venous thromboembolism (VTE) market...
Shockwave Medical announces global launch of new peripheral IVL catheter
Shockwave Medical has announced the global commercial availability of the Shockwave M5+ peripheral intravascular lithotripsy (IVL) catheter after receiving both CE mark and US...
New Janssen initiative aims to advance equitable care and address hidden...
Janssen has announced the launch of 'Save Legs. Change Lives. Spot Peripheral Artery Disease Now', a multi-year initiative aimed at creating urgency and action...
Dedicated vs. non-dedicated: Researchers place venous stenting under the spotlight at...
Venous stenting was a hot topic on the agenda of the recent American Venous Forum (AVF) annual meeting (23–26 February, Orlando, USA). Catching the audience’s...
Medtronic issues voluntary recall for subset of IN.PACT Admiral and IN.PACT...
Medtronic recently voluntarily recalled a subset of its IN.PACT Admiral and IN.PACT AV paclitaxel-coated percutaneous transluminal angioplasty (PTA) balloon catheters due to the potential...
Peripheral arterial disease symptoms differ between sexes, meta-analysis finds
In future peripheral arterial disease (PAD) research, clinicians should not consider men and women as a single population and should instead report their data...
S.M.A.R.T. Radianz vascular stent system approved for transradial use in the...
Cordis recently announced that the US Food and Drug Administration (FDA) has approved the S.M.A.R.T. Radianz vascular stent system, a self-expanding stent purposefully engineered...
Cardiovascular Systems announces first in-human experience with peripheral everolimus drug-coated balloon
Cardiovascular Systems Inc (CSI) has announced the start of enrolment in a first in-human trial of the peripheral everolimus drug-coated balloon (DCB) being developed...
InspireMD announces the inclusion of its CGuard carotid stent in CREST-2...
InspireMD has announced that its CGuard embolic prevention stent system (EPS) will be included as a device option for stenting in CREST-2 (Carotid revascularisation...
Aidoc extends scope of AI solutions for medical imaging into the...
Aidoc, a technology company that provides artificial intelligence (AI) solutions for medical imaging, including in the cardiovascular space, is extending its services beyond the...
Making the invisible visible: A clearer ‘picture’ of blood vessels in...
Johns Hopkins Medicine researchers have developed and tested a new imaging approach they say will accelerate imaging-based research in the lab by allowing investigators...
CX returns to in-person format once more in the London spring
Charing Cross (CX) chair Roger Greenhalgh welcomes the vascular community to this year's symposium, due to be held 26–28 April in London, UK, and...
SIRONA head-to-head randomised trial achieves 50% enrolment
Concept Medical recently announced that the SIRONA randomised controlled trial (RCT; Head-to-head comparison of sirolimus versus paclitaxel drug-eluting balloon angioplasty in the femoropopliteal artery)...
Regio Biosciences enters into license agreement with AstraZeneca for phase 2a...
Regio Biosciences, a Hibiscus BioVentures company, recently announced it has entered into an exclusive license agreement with AstraZeneca to further develop REG-101, a novel therapeutic acting...
Humacyte’s Human Acellular Vessel for limb salvage evaluated in multiple complex...
Humacyte today announced results from the first series of compassionate use cases of the company’s investigational Human Acellular Vessel (HAV) for the treatment of...
MedAlliance acquires Japanese partner MDK Medical
MedAlliance has acquired its partner MDK Medical, a Japanese vascular specialist company. MDK Medical has previously worked closely with MedAlliance in developing Japanese clinical...
Three-year results of Veryan Medical’s MIMICS-3D EU study revealed at ISET...
Veryan Medical has confirmed the release of MIMICS-3D EU three-year results by the study principal investigator Michael Lichtenberg (Vascular Center Arnsberg, Klinikum Hochsauerland, Arnsberg,...
Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee...
Medtronic recalls HawkOne directional atherectomy system due to risk of tip...
According to the US Food and Drug Administration (FDA), Medtronic is recalling its HawkOne directional atherectomy system.
The FDA has identified the recall as a...
Fluidx Medical reports successful use of GPX embolic device in challenging...
Fluidx Medical recently announced that its GPX embolic device was used to effectively devascularise a large tumour with multiple feeding vessels as part of...
Prolonged TV watching may increase risk of venous thromboembolism
A new study reports that watching TV for four hours a day or more is associated with a 35% higher risk of venous thromboembolism...
Novel system-wide interdisciplinary team diverts patients from amputation and improves outcomes
Findings from a first-of-its-kind study conducted at University Hospitals (UH) Harrington Heart & Vascular Institute (Cleveland, USA) showed a novel system-wide interdisciplinary team assembled...
Jeremy Durack and John Simpson to join Cordis-X innovation accelerator
Ajax Health has announced the appointments of two cardiovascular professionals to Cordis-X, an independent accelerator created to drive innovation and growth to Cordis.
"Since the...
Philips integrates cloud-based AI and 3D mapping into its mobile C-arm...
Royal Philips today announced physicians will now have access to advanced new 3D image guidance capabilities through the image-guided therapy mobile C-arm system—Zenition, aiming...
Philips integrates cloud-based AI and 3D mapping into its mobile C-arm...
Royal Philips today announced physicians will now have access to advanced new 3D image guidance capabilities through its image-guided therapy mobile C-arm system—Zenition, aiming...
Transit Scientific announces successful use of XO Cross platform in challenging...
Transit Scientific recently announced that its XO Cross platform has been successfully used in challenging peripheral vascular procedures.
A press release details that Jihad Mustapha,...
Study confirms efficiency of Upstream Peripheral’s GoBack catheter for complex lower...
Upstream Peripheral Technologies announced today that its GoBack catheter for crossing and re-entry was proven very effective for patients undergoing complex revascularisations in lower...
UK MP highlights efforts to improve care landscape for lower limb...
Emma Hardy, UK Member of Parliament (MP) for Hull West and Hessle and chair of the All-Party Parliamentary Group on Vascular and Venous Disease,...
VOYAGER PAD subanalysis highlights risks for claudicants undergoing revascularisation
Principal investigator of the VOYAGER PAD trial, Marc Bonaca (University of Colorado Anschutz Medical Campus, Aurora, USA) speaks to Vascular News about the latest data...
Large-scale IVUS analysis adds “meaningful data” to growing pool of evidence
A large-scale analysis of the use of Philips' intravascular ultrasound (IVUS) in lower extremity peripheral vascular interventions adds “meaningful data” to a growing pool...
Philips acquires Vesper Medical
Royal Philips today announced that it has signed an agreement to acquire Vesper Medical, a US-based medical technology company that develops minimally-invasive peripheral vascular...
Cordis names George Adams as chief medical officer
Cordis has announced George Adams as chief medical officer.
"We are delighted to have Dr Adams join our team as we continue to build the...
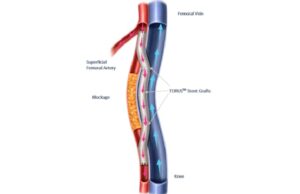
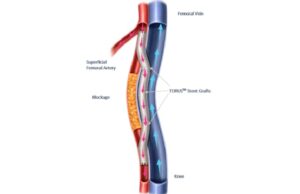
Endologix announces completion of enrolment in TORUS 2 study for PAD...
Endologix has completed enrolment in the TORUS 2 investigational device exemption (IDE) clinical study in the USA, a press release reports.
The TORUS 2...
Shockwave Medical enrols first patient in Disrupt BTK II study for...
Shockwave Medical has announced the start of the Disrupt BTK II postmarket study to assess the safety, effectiveness and optimal clinical use of the...
Avinger announces FDA clearance of Pantheris for the treatment of in-stent...
Avinger recently announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for a new clinical indication for the...
SIO announces ACCLAIM clinical trial with support from industry partners
The Society of Interventional Oncology (SIO) has announced the launch of the society’s first clinical trial—Ablation with confirmation of colorectal liver metastases (ACCLAIM) prospective...
Viz.ai launches two new AI-powered modules for pulmonary embolism and aortic...
Viz.ai has announced the US commercial launch of its AI-powered modules for pulmonary embolism and aortic disease. Debuting at VEITHsymposium 2021 (16–20 November, Orlando, USA), the...
IN.PACT Admiral DCB performs well across wide range of clinical and...
In a pooled analysis of the predictors of drug-coated balloon (DCB) effectiveness, the IN.PACT Admiral DCB (Medtronic) performed well across a broad range of...
Endologix names Matthew Thompson president and CEO
Endologix has announced the appointment of Matthew Thompson as president and chief executive officer. Thompson will also join Endologix’s board of directors. Richard Mott,...
Philips announces large-scale study outcomes on use of IVUS in peripheral...
Royal Philips today announced the results of a new large-scale real-world analysis of Centers for Medicare & Medicaid Services (CMS) data on the health...
Hispanic adults with PAD access inpatient care most often via the...
Hispanic adults hospitalised for treatment of symptoms of peripheral arterial disease (PAD) were more likely to access this care by going to the emergency...
Inari Medical announces six-month FLASH registry interim data
Inari Medical has announced positive acute and long-term interim results from the first 500 pulmonary embolism (PE) patients enrolled in the FlowTriever outcomes registry...
CMS increases hospital outpatient payment for peripheral intravascular lithotripsy
Shockwave Medical has announced that, as part of the calendar year 2022 Medicare Hospital Outpatient Prospective Payment System (OPPS) final rule, the Centers for...
Argon Medical launches novel innovations for portal vein access procedures
Argon Medical Devices has announced the launch of two portal vein access sets intended for transjugular liver access in diagnostic and interventional procedures.
The Scorpion...
FDA clears 12 new XO Cross microcatheters
Transit Scientific has announced US Food and Drug Administration (FDA) clearance of new hydrophilic-coated XO Cross microcatheters for guidewire support, exchange, and contrast media...
Researchers create novel solution for automatic measurement of maximum aortic diameter
Marie Lannelongue Hospital of Paris Saint-Joseph Hospital Group (Paris, France) and Incepto, a European specialist in artificial intelligence (AI) applied to the medical field,...
Head-to-head trial shows superiority of FemoSeal over ProGlide for peripheral interventions
Results of the randomised, prospective, multicentre STEP trial show that the FemoSeal vascular closure system (Terumo) is superior to the Perclose ProGlide suture-mediated closure...
Sanford invents Breakthrough Device for vascular disease
An investigational device invented at Sanford Health (Sioux Falls, USA) that helps high-risk vascular disease patients has been granted a Breakthrough Device designation by...
Study shows risk of major amputation in diabetics with CLTI
A retrospective study supports the use of the Wound, ischaemia, and foot infection (WIfI) classification system to predict the revascularisation benefit for diabetic patients...
Senior GP and former BMA chair says growing epidemic of non-healing...
In support of the Legs Matter Campaign’s awareness week (11–15 October), Sam Everington, a GP at the Bromley by Bow Centre in London, UK,...
Positive data for the EkoSonic endovascular system presented at VIVA 2021
Boston Scientific announced positive results for the EkoSonic endovascular system (EKOS system) during a late-breaking clinical trial presentation at Vascular Interventional Advances (VIVA) 2021...
Eighteen-month PRESTIGE BTK data presented at VIVA 2021
Eighteen-month results from the PRESTIGE below-the-knee (BTK) study were presented as a late-breaking clinical trial at Vascular Interventional Advances (VIVA) 2021 (5–7 October, Las Vegas, USA). The...
PROMISE I 24-month results “validate the benefits of the LimFlow system”
LimFlow SA has announced 24-month results from the PROMISE I study of the LimFlow percutaneous deep vein arterialisation system, confirming "excellent and sustained outcomes"...
BD announces 510(k) clearance of expanded indications for the Rotarex atherectomy...
BD recently announced it has received 510(k) clearance for expanded indications from the US Food and Drug Administration (FDA) for the Rotarex atherectomy system.
The...
Treating the dysfunctional vascular access: The expert’s low-pressure, low-dose approach
NOTE: This advertorial is ONLY available in selected countries and geographies
This advertorial, sponsored by BD, is intended for readers in the EMEA region...
First patient treated with Cardiovascular Systems’ ViperCross peripheral support catheter
Cardiovascular Systems recently announced that the first patient has been successfully treated with its ViperCross peripheral support catheter.
Billy J Kim (The Surgical Clinic, Nashville,...
Surmodics announces successful first patient uses of Sublime radial access 0.018...
Surmodics today announced the successful first clinical uses of the Sublime radial access 0.018 RX percutaneous transluminal angioplasty (PTA) dilatation catheter.
Ankur Lodha, perfomed the...
First patient enrolled in BIONETIC-I study of iliac artery treatment with...
Biotronik has announced the first patient enrolment in the BIONETIC-I study of the safety and efficacy of the Dynetic-35 cobalt chromium balloon-expandable stent system...
BIOPACT head-to-head non-inferiority randomised controlled trial completes enrolment
Biotronik is proud to announce the completion of enrolment of the investigator-initiated BIOPACT randomised controlled trial (RCT). This non-inferiority study evaluates the safety and...
Study provides new tool to assess amputation risk following popliteal vascular...
A large, multicentre cohort study provides a simple, practical method to effectively stratify patients preoperatively into low- and high-risk major amputation categories.
According to lead...
Boston Scientific announces agreement to acquire Devoro Medical
Today, Boston Scientific announced an agreement to acquire Devoro Medical, developer of the Wolf thrombectomy platform. The non-console and lytic-free Wolf technology targets and...
Japanese Selution SLR study completes enrolment
MedAlliance, with its Japanese partner MDK Medical, has completed enrolment in the clinical study of its novel sirolimus drug-eluting balloon (DEB), Selution SLR, for...
Bluegrass Vascular announces Medicare decision to assign New Technology Ambulatory Payment...
Bluegrass Vascular Technologies (Bluegrass Vascular) announced today that the Centers for Medicare & Medicaid Services (CMS) has finalised a new Healthcare Common Procedure Coding...
Biotronik expands range of peripheral introducer sheaths
Biotronik has announced the expansion of the Fortress reinforced introducer sheath line, which is now available in 7- and 8Fr-compatible sizes in the USA...
Singapore researchers develop novel 3D model to study vascular diseases
A Singapore team of scientists and clinicians from Nanyang Technological University, Singapore (NTU Singapore) and Tan Tock Seng Hospital (TTSH), have developed a three-dimensional...
Five-year ACST-2 results: Carotid artery surgery and stenting have similar long-term...
Carotid artery surgery and stenting have comparable long-term effects on fatal or disabling stroke in asymptomatic patients with severe carotid artery stenosis. That is...
Medtronic receives CE mark for 200mm and 250mm IN.PACT Admiral DCBs
Medtronic has announced the European launch of the 200mm and 250mm IN.PACT Admiral drug-coated balloons (DCBs) following CE mark approval. The product is intended...
R3 Vascular reports the initiation of its first-in-human clinical study
R3 Vascular has reported the successful initiation of its first-in-human clinical study evaluating the technical and clinical performance of the R3 Vascular Magnitude bioresorbable...
Cardiovascular Coalition urges Congress, CMS to reverse proposed Medicare cuts during...
The CardioVascular Coalition (CVC), a coalition of physicians, care providers, advocates, and manufacturers working to improve awareness and prevention of peripheral arterial disease (PAD),...
Xact Robotics announces completion of enrolment in pilot trial evaluating use...
Xact Robotics, the developer of the Xact Ace robotic system, recently announced it has successfully completed patient enrolment in the first US study evaluating...
Favourable cost-effectiveness results for endovascular SFA treatment in claudicants
From a cost-effectiveness standpoint, primary stenting of the superficial femoral artery (SFA) for the treatment of intermittent claudication can, in many countries, be used...
Hancock Jaffe presents positive two-year VenoValve data at SVS VAM 2021
Hancock Jaffe Laboratories has announced that promising two-year post-VenoValve implantation data are being presented today at the Society for Vascular Surgery’s Vascular Annual Meeting...
Five-year STABLE II results show Zenith system makes the cut for...
Joseph V Lombardi (Cooper University Hospital, Camden, USA) reported long-term outcomes favouring the continued safety and effectiveness of a composite device (proximal covered stent...
Amputation rates higher for people with PAD who are poor or...
Poverty and Black race were associated with higher rates of lower leg amputation among people with peripheral arterial disease (PAD) who live in metropolitan areas, according...
First patient enrolled in Shape Memory Medical’s AAA-SHAPE Netherlands study
Shape Memory Medical has announced the initiation of AAA-SHAPE Netherlands, the company’s prospective, multicentre early feasibility study of the Impede-FX RapidFill device when used...
FDA approves expanded PAD indication for Xarelto plus aspirin to include...
The Janssen Pharmaceutical Companies of Johnson & Johnson today announced that the US Food and Drug Administration (FDA) has approved an expanded peripheral arterial...
VOYAGER PAD: Rivaroxaban plus aspirin should be considered after lower extremity...
“A strategy of adding rivaroxaban 2.5mg twice daily to aspirin should be considered after lower extremity bypass regardless of conduit type,” concluded Nicholas Govsyeyev...
Fist Assist device achieves significant perforator vein dilation for improved endoAVF...
Fist Assist Devices has announced that data associated with the p-FACT cohort, a subset of the recently completed, non-significant risk Fist Assist clinical trial...
Research letter highlights low adoption of supervised exercise therapy for PAD
A research letter published in Circulation: Cardiovascular Quality and Outcomes highlights a “very low” utilisation of supervised exercise therapy (SET) in symptomatic peripheral arterial...
PRISTINE registry with Selution SLR sirolimus drug-eluting balloon completes enrolment
MedAlliance has announced completion of patient enrolment in the PRISTINE clinical trial with the Selution SLR 018 drug-eluting balloon (DEB) for the treatment of...
AHA scientific statement provides update on epidemiology, diagnosis, and management of...
The American Heart Association (AHA) has released a new scientific statement on lower extremity peripheral arterial disease (PAD), focusing on contemporary epidemiology, management gaps,...
Boston Scientific initiates randomised controlled trial for the EkoSonic endovascular system
Boston Scientific has commenced enrolment in the HI-PEITHO clinical trial, a collaborative research study with the Pulmonary Embolism Response Team (PERT) Consortium and the...
Researchers recommend “timely interventions by specialists and guideline-based treatment” to reduce...
A population-based study from 2013 to 2015 in Germany has found that nearly one fifth of patients with peripheral arterial disease (PAD) did not...
Philips receives FDA Breakthrough Device designation for laser-assisted IVC filter removal...
Royal Philips today announced the US Food and Drug Administration (FDA) has granted Breakthrough Device designation for a laser-assisted inferior vena cava (IVC) filter...
Higher risk of periprocedural hazards for patients undergoing carotid interventions after...
A systematic review and meta-analysis has demonstrated that patients undergoing carotid interventions after thrombolysis have a higher risk of periprocedural hazards, compared with those...
Endologix receives FDA Breakthrough Device designation for ChEVAS system
Endologix today announced the company’s ChEVAS (chimney endovascular aneurysm sealing) system has been granted a Breakthrough Device designation from the US Food and Drug...
Terumo introduces Azur vascular plug and PG Pro peripheral microcatheter embolisation...
Terumo Medical Corporation has announced today the introduction of its Azur vascular plug. The addition to Terumo's embolisation portfolio is indicated for use to...
Nationwide study identifies sex disparities in long-term mortality after paclitaxel exposure
A German claims-based cohort study has revealed that—in 13,204 patients treated with a paclitaxel-coated device for peripheral arterial disease (PAD)—mortality differences were mostly attributable...
Sectoral Asset Management makes US$9 million investment into Cagent Vascular
Cagent Vascular recently announced the investment of US$9 million by Sectoral Asset Management. Along with the investment, Marc-Andre Marcotte has joined the board of...
Case report: Recanalisation of a long SFA occlusion with Ultrascore™ Focused...
This advertorial, sponsored by BD, is intended for healthcare professionals in Europe only.
“Improving vessel compliance with focused force longitudinal plaque fracture seems to be...
Arteriovenous fistulas contribute to higher survival of haemodialysis patients with COVID-19
A new study, published online in The Journal of Vascular Access (JVA), suggests that arteriovenous fistulas (AVFs) contribute to higher survival of haemodialysis patients...
Physicians call for clinical application of “helpful, meaningful” AMPREDICT decision support...
Researchers have found that the AMPREDICT decision support tool (DST) demonstrates “strong usability characteristics and clinical relevance” in amputation level decision making for patients...
AngioDynamics receives 510(k) clearance for AlphaVac mechanical thrombectomy system
AngioDynamics recently announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the AlphaVac mechanical thrombectomy system.
According to...
Philips announces positive two-year data from TOBA II BTK clinical trial
Royal Philips recently announced positive two-year results from the TOBA (Tack optimised balloon angioplasty) II below-the-knee (BTK) clinical trial.
The data show the Philips...
SoundBite Medical announces use of its Active Wire 0.014″ platform at...
SoundBite Medical Solutions recently announced the use of its novel Active Wire 0.014” platform at a first site in the USA in the successful...
Study provides new insights on COVID-19 risk in patients receiving dialysis
Many individuals with kidney failure have been unable to self-isolate during the COVID-19 pandemic because they require dialysis treatments in clinics several times a...
FDA clears Koya Medical’s Dayspring compression system for lower extremities
Koya Medical announced today that it has received US Food and Drug Administration (FDA) 510(k) clearance for its active compression therapy system Dayspring for...
XO Score scoring sheath platform wins medical design award
Transit Scientific today announced that its XO Score scoring sheath platform has been named a winner in the 2021 Medical Design Excellence Awards (MDEA)....
Concept Medical releases status updates on SIRONA RCT
Concept Medical has released a series of status updates on their head-to-head SIRONA (Sirolimus versus paclitaxel drug-eluting balloon angioplasty in femoropopliteal diseases) randomised controlled...
One-year outcomes from PROMISE I US study of LimFlow system published
LimFlow SA recently announced the publication of 12-month data from the full patient cohort in its PROMISE I study of the LimFlow percutaneous deep...
ACC.21: SAFE-PAD finds no increased risk of death with drug-coated devices...
Researchers have found no statistically significant difference in mortality between patients treated with drug-coated devices and non-drug-coated devices in the SAFE-PAD study. Eric Secemsky...
EuroPCR 2021: Late-breaking data demonstrate long-term benefits of Medtronic radiofrequency renal...
Medtronic today announced new clinical data from the Global SYMPLICITY registry (GSR) indicating that renal denervation with the Medtronic Symplicity renal denervation system was...
Study supports tunnelled haemodialysis catheter use for permanent dialysis access
In a recent study, Victor Demaerel (University Hospitals Leuven, Leuven, Belgium) and colleagues found that tunnelled haemodialysis catheter (THC) survival in 352 patients was...
XO Score scoring sheath platform earns CE mark approval
Transit Scientific recently announced it has received CE mark clearance in the European Union for the XO Score scoring sheath platform to facilitate dilation...
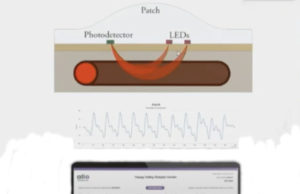
Wearable device for AV fistula remote monitoring shows promise—but faces commercial...
A new wearable device designed to remotely monitor arteriovenous (AV) fistulae function in dialysis access patients uncovered promising data leading one set of researchers...
iVascular receives CE mark for iCover covered stent
iVascular has received CE mark approval for its balloon-expandable covered stent, iCover, a press release from the company states.
Balloon-expandable covered stents are commonly used...
Micro Medical Solutions receives FDA breakthrough device designation for MicroStent vascular...
Micro Medical Solutions (MMS) recently announced that the US Food and Drug Administration (FDA) has granted breakthrough device designation for its MicroStent vascular stent....
Vascular Therapies announces clinical results from phase 3 randomised multicentre clinical...
Vascular Therapies recently announced results from its phase 3 clinical trial in which Sirogen showed encouraging arteriovenous fistula (AVF) outcomes in elderly end-stage renal...
BD announces enrolment in postmarket studies of the WavelinQ endoAVF system
BD announced today that enrolment has begun and the first patients have been treated in the postmarket surveillance study, CONNECT-AV.
CONNECT-AV is a prospective, single-arm,...
“Radiation will be a thing of the past”: FORS in the...
Opening the Charing Cross (CX) 2021 Digital Edition (19–22 April, online), experts deliberated crucial controversies in the abdominal aortic space. Discussion emphasised the potential...
CX audience supports call to change agency recommendations regarding paclitaxel use...
Addressing representatives from the US Food and Drug Administration (FDA) and UK Medicines and Healthcare products Regulatory Agency (MHRA) directly, Thomas Zeller (Bad Krozingen,...
Soundbite Medical Solutions announces FDA approval for SoundBite peripheral crossing system
Soundbite Medical Solutions has announced US Food and Drug Administration (FDA) 510(k) approval for the SoundBite crossing system—Peripheral (SCS-P) with the 0.014” active wire...
Superficial tunnelling technique not associated with patency or amputation in patients...
The results of a recent investigation have revealed no association between infrainguinal bypass tunnelling technique and primary outcomes in patients with limb ischaemia. “Compared...
Two-year IN.PACT AV Access results presented at CX 2021
Medtronic recently announced the safety and effectiveness results through 24 months for the IN.PACT AV Access clinical study. The data, which were presented virtually...
One-year results published from B Braun’s CONSEQUENT study
One-year findings from B Braun’s CONSEQUENT ALL COMERS observational study were recently published by principal investigator Ralf Langhoff (Sankt Gertrauden Hospital, Berlin, Germany) et...
Endologix announces acquisition of PQ Bypass
Endologix recently announced it has completed the acquisition of PQ Bypass, a medical technology company pioneering a first-of-its-kind technology that addresses an unmet need...
Inari Medical announces first patient enrolled in FLAME study
Inari Medical has announced the enrolment of the first high-risk pulmonary embolism (PE) patient in the FLAME (FlowTriever for acute massive pulmonary embolism) study....
Philips SmartCT application software receives FDA 510(k) clearance
Royal Philips today announced US Food and Drug Administration (FDA) 510(k) clearance for its Philips SmartCT application software. SmartCT is a key component of...
Surmodics announces first patient use of two Sublime radial access platform...
Surmodics recently announced the successful first uses in patients for two devices within its Sublime radial access platform: the Sublime radial access guide sheath...
CX to highlight impact of high-flow arteriovenous fistulas in Vascular Access...
Nicholas Inston (Queen Elizabeth Hospital Birmingham, Birmingham, UK) speaks to Vascular News ahead of this year’s Charing Cross Symposium (CX), which is being held online 19–22 April. In...
“A reliable tool”: Positive findings for 2D perfusion angiography in CLTI...
Researchers suggest that, in patients with chronic limb-threatening ischaemia (CLTI), two-dimensional (2D) perfusion angiography is a “reliable tool” when used according to standardised methods....
Shape Memory Medical receives PMDA approval for the Impede-FX embolisation plug
Shape Memory Medical recently announced that it has received approval from the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) to market its Impede-FX embolisation...
LimFlow receives Japan PMDA approval for clinical study of minimally-invasive technology
LimFlow announced today that the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has approved its Clinical Trial Notification (CTN) for the Japanese cohort of...
Cardiovascular Systems announces acquisition of peripheral support catheters From WavePoint Medical
Cardiovascular Systems recently announced that it has acquired a line of peripheral support catheters from WavePoint Medical.
Peripheral support catheters are used during peripheral vascular...
Penumbra announces US commercial availability of Indigo system Lightning 7
Penumbra today announced US commercial availability of the Indigo system Lightning 7. Lightning 7 expands Penumbra’s offering of the Indigo aspiration system with Intelligent...
MedAlliance raises over US$50 million to roll out Selution SLR and...
MedAlliance recently announced that it has raised over US$50 million in equity funding. The new investor is Trustar Capital (formerly known as CITIC Capital...
Case report: Complex endovascular treatment for an iliac artery occlusion using...
This advertorial, sponsored by BD, is only available in selected countries and geographies.
Martin Schroeder—a vascular surgery specialist at the Ruhr University Bochum Marienhospital...
Cardinal Health signs definitive agreement to sell its Cordis business to...
Cardinal Health recently announced that it has signed a definitive agreement to sell its Cordis business to Hellman & Friedman (H&F) for approximately US$1...
Shape Memory Medical receives CE mark for Impede-FX RapidFill
Shape Memory Medical has announced CE mark approval for the Impede-FX RapidFill device, an expansion of the Impede family of biodegradable peripheral vascular embolisation...
PAD-scan deemed cost-effective for PAD detection in patients with diabetes
Point-of-care duplex ultrasound (PAD-scan) is a cost-effective test for the detection of peripheral arterial disease (PAD) in patients with diabetes. This is the conclusion...
Renal artery involvement in EVAR linked to permanent dialysis and lower...
Renal artery involvement (RAI) in endovascular aneurysm repair (EVAR) is “highly predictive of the need for postoperative and permanent dialysis,” Anastasia Plotkin (University of...
First patients enrolled in the Merit Wrapsody AV access efficacy pivotal...
Merit Medical Systems recently announced the enrolment of the first patients in its Wrapsody arteriovenous (AV) access efficacy pivotal (WAVE) study of the Wrapsody endovascular...
Humacyte to present long-term follow-up data from Phase 2 vascular access...
Humacyte has announced that the clinical results of five-year outcomes in patients receiving the human acellular vessels (HAV) for arteriovenous (AV) access in haemodialysis...
Medtronic launches IN.PACT AV DCB in Japan for patients undergoing haemodialysis
Medtronic has announced the launch of IN.PACT AV drug-coated balloon (DCB) in Japan. IN.PACT AV DCB is indicated for the treatment of obstructive lesions...
Meta-analysis: IVC filters should be considered for certain patients at high...
In a recent meta-analysis, Yang Liu, Huan Lu (Henan Cancer Hospital, Zhengzhou, China), and colleagues found insufficient evidence to prove that inferior vena cava...
FDA advisory panel recommends against premarket approval of Lutonix 014 DCB...
A US Food and Drug Administration (FDA) advisory panel has recommended against premarket approval of BD’s Lutonix 014 drug-coated balloon (DCB) for use in...
HydroMID midline catheter from Access Vascular receives FDA clearance
Access Vascular has announced US Food and Drug Administration (FDA) clearance of its HydroMID midline catheter.
A press release from the company states that...
PatenSee initiates first-in-human trial with its contactless imaging surveillance system for...
PatenSee has initiated a first-in-human clinical trial to assess its machine vision-based surveillance system, a press release from the company reveals. The trial, led...
Real-world data on SeQuent Please OTW DCB presented at LINC 2021
One-year findings of the CONSEQUENT all-comers observational study of the paclitaxel drug-coated balloon (DCB) SeQuent Please OTW (B Braun) were reported at LINC 2021...
New standardised reporting system suggests arteriovenous graft infections may be less...
“Using an objective system that discriminates between aetiology and outcome allows a more complete, objective understanding of relative infection risks and outcomes for arteriovenous...
Soundbite announces Health Canada approval for SoundBite crossing system – Peripheral...
Soundbite Medical Solutions has announced Health Canada approval for the SoundBite Crossing System – Peripheral (SCS-P) with the 0.014” Active Wire (14P).
The SCS-P (14P)...
LINC 2021: Novel approaches and new data for BTK interventions revealed
In a late-breaking trial session at LINC 2021 (The Leipzig Interventional Course, 25–29 January, online), key updates on below-the-knee (BTK) interventions were in the...
Transit Scientific announces close of its Series A financing round
Transit Scientific has announced the close of its Series A financing round. The round was led by a large multinational investor and joined by...
Novel embolic device used for arterial and venous tumour treatments
Fluidx Medical Technology has announced that GPX Embolic Device first patient use cases were presented at LINC 2021 (25–29 January, online).
“This is very promising...
VasQ external support awarded NUB Status 1 reimbursement renewal for 2021...
The German Institute for Hospital Remuneration (InEK) has renewed the new examination and treatment methods (NUB) Status 1 designation of VasQ external support (Laminate...
LINC 2021: PRELUDE-BTK results confirm Serranator device novel mechanism of action
Cagent Vascular announced the results of its PRELUDE-BTK study at LINC 2021 (25–29 January, online). The PRELUDE-BTK study was a prospective, single-arm, multicentre feasibility...
CLEAN3 trial outlines potential new best practices for peripheral IV catheters
BD today announced that new clinical data have found robust evidence that using a vascular care solution can help improve outcomes for patients requiring...
BIOLUX AV trial demonstrates safety and efficacy of Passeo-18 Lux DCB...
Recent data from the investigator-initiated, randomised controlled trial (RCT), BIOLUX AV, showed that the treatment of patients with dysfunctional haemodialysis access with BIOTRONIK's Passeo-18...
IVUS should be considered “essential equipment” in venous interventions, LINC 2021...
“I consider intravascular ultrasound (IVUS) to be essential equipment in venous interventions,” Rick de Graaf (Clinic of Friedrichshafen, Friedrichshafen, Germany) told viewers of LINC...
LINC 2021: Long-term data show sustained efficacy and safety of paclitaxel...
A key theme among the late-breaking trial data presented at LINC 2021 (The Leipzig Interventional Course; 25–29 January, online) was the emergence of long-term...
LINC 2021 first time data releases in arteriovenous access: Positive results...
New data presented for the first time at LINC 2021 (The Leipzig Interventional Course; 25–29 January, online) are positive for the IN.PACT AV drug-coated...
LINC 2021: 12-month BTK data with MedAlliance’s SELUTION SLR presented as...
Twelve-month results from the PRESTIGE below-the-knee (BTK) study have been presented as a late-breaking trial at LINC 2021 (25–29 January, virtual). The objective of...
Artio Medical announces successful first human use of the Amplifi vein...
Artio Medical recently announced that it has successfully completed the first human use of its Amplifi vein dilation system. The first clinical procedure was...
Three studies evaluating Flex Vessel Prep system presented during LINC 2021
VentureMed Group announced today that data from three studies evaluating the use of its Flex Vessel Prep (VP) system were presented during LINC 2021...
LINC 2021: Head-to-head trials take centre stage in drug-eluting technology late-breaking...
Data from two head-to-head trials on drug-eluting technologies in femoral artery treatment were presented during a late-breaking session at LINC 2021 (Leipzig Interventional Course;...
Five-year ILLUMENATE results confirm safety profile of Philips Stellarex DCB
Royal Philips has announced the final, five-year results of two randomised controlled trials (RCTs) that show no difference in all-cause mortality between patients treated...
Study: Patients with depression less likely to go home after critical...
A recent analysis by finds an association between depression and non-home discharge after revascularisation for chronic limb-threatening ischaemia (CLTI). Authors Joel L Ramirez, James...
Largest US nephrology group is first in Illinois to offer Ellipsys...
Nephrology Associates of Northern Illinois and Indiana (NANI), the largest nephrology group in the USA, is the first practice in Illinois to adopt the Ellipsys vascular access system (Avenu Medical, now...
“From pioneering times to a mature technology”: The evolution of bridging...
There are several covered stents on the market that are used in an off-label setting as bridging stents for fenestrated and branched endovascular aneurysm...
Mermaid Medical Group reports full commercialisation of the D-Clot HD rotational...
Mermaid Medical Group recently announced US commercial availability of the D-Clot HD rotational thrombectomy system. As of 12 January, the company reports 25 successful...
Arterial diameter may help to predict AVF aneurysm progression, study suggests
A recent study concludes that arterial diameter may influence arteriovenous fistula (AVF) aneurysm progression and the interval to surgical revision. “Patients with larger arterial...
Network meta-analysis finds DCB angioplasty “significantly superior” to plain balloon angioplasty...
A recent network meta-analysis shows that, in failing arteriovenous fistulas (AVFs) with outflow stenosis, drug-coated balloon (DCB) angioplasty is “significantly superior” to plain balloon...
SWEDEPAD unplanned interim analysis shows no difference in all-cause mortality for...
An unplanned interim analysis of the registry-based SWEDEPAD clinical trial, in which patients with peripheral arterial disease received treatment with paclitaxel-coated (drug-coated balloons or...
Sirtex Medical announces strategic investment in BlackSwan Vascular
Sirtex Medical (Sirtex) has announced a strategic investment in BlackSwan Vascular (BlackSwan), a private company developing therapies in endovascular embolization. The investment provides resources...
COVID-related hypercoagulability linked to elevated malfunction rate in temporary haemodialysis catheters
Hypercoagulability in COVID-19 patients leads to an increase in the malfunction rate of temporary haemodialysis catheters—but heparin locking of the catheters is linked to...
Twelve-month LOCOMOTIVE EXTENDED results “promising”
B Braun has revealed that 12-month results from the LOCOMOTIVE EXTENDED study of the company’s Multi-Loc multiple stent delivery system have been published in...
First-in-human study demonstrates “feasibility and potential” of FORS technology
Based on the findings of their first-in-human feasibility study, Joost van Herwaarden (Utrecht University Medical Centre, Utrecht, The Netherlands) and colleagues write in the...
Risk of ischaemic steal syndrome and patency rate comparable for tapered...
In their recent systematic review and meta-analysis, Venkata Sai Jasty (University of Arizona, Tuscon, USA) and colleagues found that the risk of ischaemic steal...
ISABELLA trial for the treatment of failing AV fistulas in haemodialysis...
MedAlliance has announced completion of patient enrolment in the ISABELLA clinical trial with the Selution SLR 018 drug-eluting balloon (DEB) for the treatment of...
Penumbra’s newest generation of Indigo aspiration system receives FDA clearance for...
Penumbra today announced US Food and Drug Administration (FDA) 510(k) clearance for expanded indication of the latest iteration of the Indigo aspiration system, Lightning...
Paclitaxel situation “crystallising” thanks to new data and teamwork, VIVA audience...
This year's Vascular Interventional Advances annual meeting (VIVA 2020; 6–8 November, online) opened with a session on controversial hot topics and advanced multidisciplinary approaches...
Infinity angioplasty balloon catheter completes first-in-human clinical trial
The Infinity Angioplasty Balloon Company announced today that its angioplasty balloon platform—the Infinity angioplasty balloon catheter—has successfully completed a first-in-human clinical trial.
The platform was...
First procedures performed with Transit Scientific’s XO Score angioplasty system
According to a press release, Transit Scientific's XO Score system has been successfully used to dilate multiple challenging fibrotic and calcific lesions in haemodialysis...
AngioDynamics announces presentation of positive safety, efficacy results from RAPID outcomes...
AngioDynamics has announced the safety and efficacy results from the RAPID (Registry of AngioVac system procedures in detail) database. Results were shared by principal...
No safety concerns and favourable patency at two years with Absorb...
The Absorb bioresorbable vascular scaffold (BVS; Abbott Vascular) can be used for the treatment of chronic limb-threatening ischaemia (CLTI) patients in infrapopliteal arteries with...
Six-month data from Surmodics Avess AV fistula DCB first-in-human study presented...
Surmodics recently announced that six-month data from the AVESS first-in-human (FIH) study of the company’s Avess arteriovenous fistula (AVF) drug-coated balloon (DCB) was shared...
DETOUR I two-year outcomes: “Excellent” functional improvement in complex PAD cohort
During a late-breaking data session at this year’s Vascular Interventional Advances annual meeting (VIVA 2020; 6–8 November, virtual), Ehrin Armstrong (University of Colorado, Denver,...
RCT investigating use of MagicTouch PTA sirolimus-coated balloon for PAD enrols...
Concept Medical has announced the enrolment of the first patient in the SirPAD (Sirolimus in peripheral arterial disease) trial. According to a press release,...
PROMISE I: LimFlow system enables vast majority of patients to avoid...
LimFlow SA has announced the presentation of one-year data from the full patient cohort in its PROMISE I study of the LimFlow percutaneous deep...
Similar outcomes for ultrasound-assisted and standard thrombolysis in SUNSET sPE
Efthymios Avgerinos (University of Pittsburgh, Pittsburgh, USA) presented results from the SUNSET sPE trial during a late-breaking data session at this year’s Vascular Interventional...
Safety and efficacy of IN.PACT Admiral sustained out to five years
In a late-breaking data session at this year’s Vascular Interventional Advances conference (VIVA 2020; 6–8 November, virtual) Thomas Zeller (University Heart Center Freiburg, Bad...
One-year VIVO results support safety and effectiveness of Zilver Vena venous...
Results of the VIVO clinical study support the safety and effectiveness of Cook Medical's recently FDA-cleared Zilver Vena venous stent for the treatment of...
Directional atherectomy prior to IN.PACT DCB effective out to one year,...
A vessel preparation treatment strategy of directional atherectomy prior to drug-coated balloon angioplasty with Medtronic’s IN.PACT Admiral drug-coated balloon (DCB) in long, calcified femoropoliteal...
Latest VOYAGER PAD analysis finds no mortality and improved limb outcomes...
Given the absence of a safety signal in data from the VOYAGER PAD trial, a new analysis examined the potential benefit of drug-coated device...
DISRUPT PAD III: Intravascular lithotripsy superior to PTA in acute procedural...
The DISRUPT PAD III randomised controlled trial (RCT) provides the largest level one evidence for the treatment of heavily calcified femoropopliteal arteries, noted William...
Four-year results from patient-level meta-analysis confirm safety profile of Philips Stellarex...
Royal Philips today announced the results of a patient-level meta-analysis that confirms the safety profile of its Stellarex drug-coated balloon (DCB) at four years....
Boston Scientific receives FDA approval for the Ranger DCB
Boston Scientific announced it has received US Food and Drug Administration (FDA) approval of the Ranger drug-coated balloon (DCB), developed for the treatment of...
Case report shows Ellipsys system creates fused, permanent vascular access for...
A new case report published in the Journal of Vascular Surgery (JVS) provides one of the first known opportunities to directly visualise the anastomosis...
Medtronic Abre stent receives US FDA approval to treat venous outflow...
Medtronic today announced it has received US Food and Drug Administration (FDA) approval for the Abre venous self-expanding stent system. This device is indicated...
Janssen submits application to US FDA for new indication to expand...
The Janssen Pharmaceutical Companies of Johnson & Johnson announced today it has submitted a supplemental New Drug Application (sNDA) to the US Food and...
Study finds 3mm arteriotomy may be routinely utilised for brachiocephalic fistula...
In a recently-published study, Jeremy Crane (Imperial College Healthcare NHS Trust, Hammersmith Hospital, London, UK) and colleagues conclude that a 3mm-long arteriotomy may be...
Endovascular late-breaking trials presented at TCT 2020
Findings from a series of late-breaking trials in the endovascular field were presented recently at TCT Connect (14–18 October, virtual), the 32nd annual scientific...
Zilver Vena venous self-expanding stent receives FDA approval to market in...
Cook Medical today announced that the Zilver Vena received US Food and Drug Administration (FDA) premarket approval (PMA) in the USA. The product is...
Ra Medical Systems announces 10 subjects have been enrolled in its...
Ra Medical Systems has announced enrolment of the tenth subject in its pivotal clinical trial to evaluate the safety and effectiveness of the Dabra...
Clinician survey reveals significant variation in ultrasound-guided PIV insertion
A new survey among vascular access (VA) and emergency department (ED) clinicians has revealed significant levels of variation in ultrasound-guided peripheral IV (UGPIV) practices...
First two patients enrolled in PRISTINE study with Selution SLR
MedAlliance has announced enrolment of the first two patients in the PRISTINE registry with Selution sustained limus release (SLR) 018 drug-eluting balloon (DEB) for...
Reflow Medical receives approval in Japan for the Wingman catheter
Reflow Medical has announced that Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) has approved the Wingman chronic total occlusion (CTO) catheter. Reflow Medical has...
PQ Bypass completes enrolment in DETOUR II pivotal study
PQ Bypass has announced enrolment of the final subject in the company’s DETOUR II investigational device exemption (IDE) clinical trial. This milestone occurs only...
Interventionalists encouraged to consult talking points document on paclitaxel devices
Interventionalists are being encouraged to take consideration of a set of talking points about the risks and benefits of paclitaxel-equipped devices—which has developed by...
Teleflex announces expanded indications for the Arrow EZ-IO intraosseous vascular access...
Teleflex has announced it has received 510(k) clearance from the US Food and Drug Administration to expand the indications for use of the Arrow...
Medtronic to acquire Avenu Medical
Medtronic today announced the planned acquisition of Avenu Medical, a medical device company focused on the endovascular creation of arteriovenous (AV) fistulae for patients...
CIRSE 2020: New clinical data support use of 4F devices for...
Outcomes of the BIO4AMB trial demonstrated that ambulatory treatment with 4-French (4F) devices is a valid and safe option for endovascular treatment of lower...
Ellipsys system offers greater patient eligibility and reduced time to dialysis,...
Two devices for creating minimally invasive dialysis access—the Ellipsys vascular access system (Avenu Medical) and the WavelinQ 4F system (BD)—demonstrated high rates of technical...
Dialysis access needs to be a priority in the COVID-19 era
Panagiotis M Kitrou and Nicholas Inston discuss the impact of the COVID-19 pandemic on vascular access services. Despite the disruption caused, they are optimistic...
First patient enrolled in FUTURE SFA study of MagicTouch sirolimus-coated balloon
Concept Medical has announced the enrolment of the first patient in the FUTURE SFA (Randomised controlled trial of first sirolimus coated balloon versus standard...
CMS grants additional reimbursement for the Eluvia drug-eluting vascular stent system
Boston Scientific announced that the US Centers for Medicare and Medicaid Services (CMS) granted a new technology add-on payment (NTAP) for the Eluvia drug-eluting...
Study finds Ellipsys system allows kidney patients to begin dialysis sooner...
The Ellipsys vascular access system reduces the time before patients with kidney failure can start lifesaving dialysis treatments, while requiring fewer secondary procedures, according...
PQ Bypass receives FDA breakthrough device designation for the Detour system
PQ Bypass has announced that they received breakthrough device designation from the US Food and Drug Administration (FDA) for the Detour system. The Detour...
Patient-specific computation model may improve AVF maturation rates
The results of a randomised controlled trial—the Shunt simulation study—show that a new patient-specific computation model accurately calculated postoperative access flow. As flow is...
Inaugural CX Aortic Vienna to take place online
The inaugural CX Aortic Vienna meeting will be livestreamed 8–11 September 2020 to an international, online audience, and will include registrant participation, interaction, and...
Systematic review and meta-analysis finds “substantial” one-year mortality rate in octogenarians...
In octogenarians with chronic limb-threatening ischaemia (CLTI), researchers found a one-year mortality rate of 32% after revascularisation, which was significantly higher than in non-octogenarians....
Enrolment initiated in world’s first RCT with sirolimus-coated balloon for below-the-knee...
Concept Medical has announced the enrolment of the first patient in the FUTURE BTK (Randomised controlled trial of first sirolimus coated balloon versus standard...
Philips to expand its image-guided therapy portfolio through acquisition of Intact...
Royal Philips today announced that it has signed an agreement to acquire Intact Vascular. According to a press release, Intact Vascular will enhance Philips’...
High level of heterogeneity found in randomised PAD trial antithrombotic regimens
A recent systematic review showed that randomised trials comparing different types of peripheral endovascular arterial intervention have a high level of heterogeneity in their...
First patient enrolled in PATHFINDER I registry examining Auryon atherectomy system
AngioDynamics has announced the enrolment of the first patient in the PATHFINDER I postmarket registry.
The PATHFINDER I registry is a pilot study to evaluate...
Six-month IN.PACT AV Access results show superiority of DCB angioplasty
Just-published six-month results of the IN.PACT AV Access study show that drug-coated balloon (DCB) angioplasty is superior to standard angioplasty for the treatment of...
First patients enrolled in SUPERSURG study of Supera peripheral stent system
ID3 Medical Belgium has announced the first two enrolments in the SUPERSURG study to investigate the safety and efficacy of Abbott's Supera peripheral stent...
Regional anaesthesia alone is reasonable for major lower extremity amputation in...
A recent study, published online on 3 August in the European Journal of Vascular and Endovascular Surgery (EJVES), found that regional anaesthesia alone is...
Compression after radiofrequency ablation of varicose veins “adds no clinical benefit”
A randomised controlled trial has found that clinical and patient-reported outcomes following radiofrequency ablation (RFA) of varicose veins without compression are no worse than...
Endologix receives CE mark for Alto abdominal stent graft system
Endologix recently announced that it has received a CE mark for the Alto abdominal stent graft system.
“We are very excited to receive a CE...
Surmodics receives FDA 510(k) clearance for Sublime radial access 0.014 RX...
Surmodics recently announced it has received US Food and Drug Administration (FDA) 510(k) clearance for its Sublime radial access 0.014 rapid exchange (RX) percutaneous...
Artio Medical acquires Flow Forward Medical, expanding peripheral vascular portfolio
Artio Medical today announced it has acquired Flow Forward Medical, a medical device company developing methods for establishing and maintaining vascular access sites.
This...
Balloon-expandable covered stent reveals promise as endovascular alternative to surgery in...
This advertorial is sponsored by Bentley.
Maria Antonella Ruffino, a vascular interventional radiologist at Azienda Ospedaliera Universitaria Città della Salute e della Scienza in Turin,...
First sirolimus DEB patient enrolled in Japanese SELUTION SLR study
MedAlliance’s partner in Japan, MDK Medical, has enrolled the first patient in the clinical study of its novel sirolimus drug-eluting balloon (DEB), SELUTION SLR,...
New study shows advantages of Ellipsys system in creating reliable dialysis...
A new study shows significant benefits of the Ellipsys vascular access system (Avenu Medical) in easily and safely creating durable vascular access for end-stage...
Veryan Medical to support commercialisation of Jeti thrombectomy system in Germany
It was recently announced that Veryan Medical will support Walk Vascular in the commercialisation of the Jeti peripheral thrombectomy system in Germany.
Walk Vascular’s Jeti...
UK-first innovation could save lives of dialysis patients
Barts Health NHS Trust has performed a UK-first operation outside of trial setting that could reduce mortality and risks of infection in patients with...
Advance Serenity hydrophilic PTA balloon catheter now available in the USA
Cook Medical recently announced that the Advance Serenity hydrophilic percutaneous transluminal angioplasty (PTA) balloon catheter is now available to physicians in the USA. The...
New data reconfirm long-term safety profile of the Philips Stellarex low-dose...
Royal Philips recently announced the four-year results from the ILLUMENATE European randomised controlled trial (EU RCT). The Stellarex drug-coated balloon (DCB) cohort demonstrated similar...
MERLION trial six-month outcomes presented at VIVA late-breaking trial session
Six-month clinical outcomes of the MERLION trial were presented as part of the VIVA Late-Breaking Clinical Trials Livesteam (25 June), by Tjun Tang (Singapore...
iVascular launches Sergeant peripheral support catheter
iVascular recently announced the launch of its Sergeant peripheral support catheter. Sergeant is a CE marked over-the-wire catheter, indicated for patients with peripheral arterial...
Intact Vascular announces positive one-year data from TOBA II BTK clinical...
Intact Vascular announced the positive one-year results from its Tack optimised balloon angioplasty (TOBA) II BTK clinical trial during the 2020 Vascular Interventional Advances (VIVA)...
Real-world analysis demonstrates lower long-term mortality after DCB angioplasty of femoropopliteal...
In a real-world retrospective analysis, recently published in JACC: Cardiovascular Interventions, the long-term mortality rate was lower after drug-coated balloon (DCB) angioplasty than after plain...
INFINITY Angioplasty Balloon Catheter receives 510(k) clearance
The Infinity Angioplasty Balloon Company has announced that its balloon platform—the INFINITY Angioplasty Balloon Catheter—has been cleared by the US Food and Drug Administration...
Surmodics receives CE mark for its SurVeil drug-coated balloon
Surmodics recently announced it has received CE mark certification in the European Union for its SurVeil drug-coated balloon (DCB).
“I am excited about the potential...
MHRA: Warning to be added to paclitaxel device IFUs in Europe
In a new field safety notice, the UK Medicines and Healthcare products Regulatory Agency (MHRA) states that a warning and clinical summary section will...
Lipidol Ultra-Fluid recevies approval for use in cTACE by China NMPA
Guerbet has announced the approval of an additional indication for Lipiodol Ultra-Fluid by the China NMPA (National Medical Products Administration) for use in transarterial...
CX 2020 LIVE: Vascular Access session showcases new technologies, while debate...
The CX 2020 LIVE Vascular Access Consensus session sparked global interest, with chair Nick Inston (Birmingham, UK) and moderator Domenico Valenti (London, UK) taking...
CX 2020 LIVE: Strong support for relining peripheral arteries and rivaroxaban use for widespread atherosclerosis
In a series of Podium 1st presentations from world-class faculty, CX 2020 LIVE attendees heard the latest data on peripheral arterial disease management and an evaluation of different types of stents, including...
Shockwave Medical announces that CMS has created new codes for intravascular...
Shockwave Medical announced today that the Centers for Medicare & Medicaid Services (CMS) has issued new codes for intravascular lithotripsy (IVL) procedures performed in...
CX 2020 LIVE aortic arch discussion highlights importance of underlying pathology...
Last week week, the CX 2020 LIVE agenda turned to the technically challenging topic of aortic arch interventions. Through presentations, discussion, and polling, the...
CX 2020 LIVE gains CME accreditation: Attendees from 79 countries participate live
In a boost for vascular education, the CX 2020 LIVE sessions have been granted European Union (EU) and US reciprocal Continuing Medical Education (CME) points in recognition of its...
VasQ external support awarded breakthrough device designation by the FDA
The US Food and Drug Administration (FDA) has designated Laminate Medical’s VasQ external support for the creation of arteriovenous fistulas (AVF) in haemodialysis patients...
CX 2020 LIVE pioneers virtual vascular conference format in COVID-19 era
CX 2020 LIVE came to life online—despite COVID-19—using state-of-the art broadcast technology to bring together more than 1,000 vascular specialists, live, from 95 countries across...
Positive two-year data from ALPS registry of LimFlow system published
LimFlow SA today announced publication of positive two-year data from the ALPS registry of the LimFlow percutaneous deep vein arterialisation (pDVA) system. Results were...
Bluegrass Vascular Technologies announces first US commercial cases with the Surfacer...
Bluegrass Vascular Technologies has announced that the first US commercial cases using the Surfacer Inside-Out access catheter system were completed at Santa Clara Valley...
Getinge introduces Advanta V12 balloon expandable covered stent in larger size
Getinge recently announced the launch of its Advanta V12 large diameter stent.
According to a press release, Getinge’s Advanta V12 stent has been shown to...
Global support network “will improve the representation of women in endovascular...
While more than 60% of medical students are female, the number of women in endovascular medicine is low, report Bella Huasen and Agnieszka Solberg....
SCAI issues device selection guidelines for aortoiliac arterial interventions
The Society for Cardiovascular Angiography and Interventions (SCAI) has released guidelines that provide a comprehensive review of comparative effectiveness data for devices used in...
TOBA III clinical trial results published in the Journal of Vascular...
Intact Vascular has announced the peer-reviewed publication of its Tack optimised balloon angioplasty (TOBA) III 12-month clinical trial results in the Journal of Vascular Surgery.
The multi-centre, single-arm,...
VIVA Physicians publishes analysis evaluating mortality and paclitaxel-coated devices
An individual patient-level data (IPD) analysis of the safety of paclitaxel-containing devices (PTXD), conducted by VIVA Physicians, identified an absolute 4.6% increased mortality risk...
FDA clears Aspire MAX mechanical thrombectomy system
Control Medical Technology has announced that the US Food and Drug Administration (FDA) has cleared its Aspire MAX 7 – 11F mechanical thrombectomy...
New study addresses thromboembolic complications in COVID-19 patients
There is an “urgent need” to improve specific venous thromboembolism (VTE) diagnostic strategies and investigate the efficacy and safety of thromboprophylaxis in ambulatory COVID-19...
Global vascular community addresses geographic disparity in pandemic impact and response,...
Last week, the Charing Cross (CX) Symposium 2020 would have taken place in London, UK. To mark the occasion virtually, experts from across the...
First US commercial use of Tack Endovascular System (4F) in BTK...
Intact Vascular has announced the first commercial use of its Tack Endovascular System (4F) in multiple sites across the USA. Notably the first FDA-approved...
First patient enrolled globally in SELUTION SLR study for AV fistulae
MedAlliance has announced enrolment of the first patient in the SAVE (Use of the Selution sirolimus-eluting balloon for dysfunctional AV access treatment indications) study...
Philips and the US government collaborate in ventilator production ramp up...
Royal Philips has announced that the US government and Philips agreed to team up to increase the production of hospital ventilators in its manufacturing...
Virtual VIVA roundtable addresses COVID-19 pandemic
VIVA Physicians recently hosted a virtual roundtable to address COVID-19. Tony Das (Connected Cardiovascular Care Associates, Dallas and BSW Heart Hospital, Plano, USA), one...
ACS defends healthcare workers as PPE shortages lead to acrimony
The American College of Surgeons (ACS) today leapt to the defense of healthcare workers who are heading to work in the face of increasing...
Virtual ACC: Clopidogrel exposure along with aspirin and rivaroxaban should be...
“More bleeding with background clopidogrel, even if not severe by adjudication, may be associated with broad consequences, including discontinuation of therapies. In the absence...
CX 2020 is cancelled due to COVID-19
The 2020 Charing Cross (CX) Symposium that was due to take place next month in London, UK, has been cancelled due to COVID-19.
In full,...
Straub Medical announces first patient treated in USA with Rotarex S atherectomy device
Straub Medical (United States), the US direct-sales subsidiary of Straub Medical AG (Switzerland), has announced the launch and first intervention performed with the Rotarex S...
National Kidney Foundation releases guideline for vascular access in dialysis patients
The American Journal of Kidney Diseases (AJKD) has published the National Kidney Foundation's KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update, a completely...
SeQure® microcatheter could help to make embolisation more secure
NOTE: This video is ONLY available to watch in selected countries and geographies
Geert Maleux (Leuven, Belgium), one of the first users of the SeQure®...
Okami Medical announces first patients treated with Lobo vascular occluder
Okami Medical has announced the successful completion of the first cases with the Lobo vascular occlusion system. The first offering in the company's product...
Wingman catheter granted expanded indication to cross CTOs in PAD
Reflow Medical has announced that they have received US Food and Drug Administration (FDA) clearance for an expanded indication for the Wingman crossing catheter...
Ablative Solutions announces publication of data from the Peregrine post-market study
Ablative Solutions has announced that positive six-month results from the Peregrine post-market study demonstrating the safety and efficacy of the company’s CE-marked Peregrine System infusion...
LINC 2020: Geographical “inconsistencies” cast doubt on paclitaxel mortality signal
Peter Schneider (San Francisco, USA) talks to BLearning Peripheral at LINC 2020 (Leipzig Interventional Course; 28–31 January 2020, Leipzig, Germany) about the relevance of dose relationship and geographical...
Published real-world experience demonstrates VasQ External Support improves outcomes when adopted...
Robert Shahverdyan, head of the Vascular Access Center at Asklepios Klinik Barmbek, Hamburg, Germany, has recently published a retrospective analysis of his first 32...
VEITH 2019: “Light at the end of the tunnel” following paclitaxel...
Sean Lyden (Cleveland, USA) talks to VEITHtv at the VEITHsymposium 2019 (19–23 November, New York, USA) about the “tumultuous” year for the industry following the publication of the...
LINC 2020: Real-world evidence shows no increased mortality signal with paclitaxel-coated...
Dierk Scheinert (Leipzig, Germany) moderates a panel with Eva Freisinger (Münster, Germany) and Thomas Zeller (Bad Krozingen, Germany) to discuss the developments over the...
FDA grants de novo clearance to Bluegrass Vascular for Surfacer system
Bluegrass Vascular Technologies (Bluegrass Vascular) announced today that the US Food and Drug Administration (FDA) has granted a de novo classification order for its...
Rist Neurovascular radial artery catheter receives FDA 510(k) clearance
Rist Neurovascular recently announced that it has received US Food and Drug Administration (FDA) 510(k) clearance to market the Rist Cath Radial Access Long...
XableCath crossing catheters have received CE mark for peripheral use
XableCath has announced that its XableCath Crossing catheters have received CE mark for peripheral use. Its crossing devices will be available for sale in...
Enrolment of the VasQ external support US pivotal study now complete
Laminate Medical Technologies has announced the completion of enrolment into the VasQ external support US pivotal study. The study was conducted at 17 sites...
Medtronic begins pilot study as part of SPYRAL HTN clinical programme...
Medtronic has announced that it will begin enrolment in a pilot study evaluating the safety and efficacy of the Symplicity Spyral renal denervation (RDN)...
IVL has “changed the dynamics” of facilitating EVAR & TEVAR in...
Frank Arko (Charlotte, USA) talks to VEITHtv at the VEITHsymposium 2019 (19–23 November, New York, USA), about the benefits of using Shockwave Intravascular Lithotripsy (IVL) when performing EVAR...
MedAlliance receives CE mark approval for SELUTION SLR sirolimus drug-eluting balloon...
MedAlliance has announced the award of its first CE mark approval: for SELUTION SLR, a novel sirolimus drug-eluting balloon (DEB), for the treatment of...
LINC 2020: Four-year ILLUMENATE data reaffirm safety profile of Stellarex
Philips today announced four-year results from the randomised controlled ILLUMENATE pivotal trial in the USA. The data show similar mortality rates through four years...
LINC 2020: BD announces long-term safety data for BTK drug-coated balloon
Today at the 2020 Leipzig Interventional Course (LINC; 28–31 January, Leipzig, Germany), BD announced that interim findings from the Lutonix below-the-knee (BTK) investigational...
LINC 2020: High- and low-dose paclitaxel-coated balloons exhibit comparable results at...
At the 2020 Leipzig Interventional Course (28–31 January, Leipzig, Germany), Sabine Steiner (University of Leipzig, Leipzig, Germany) presented one-year results of the COMPARE-RTC of...
LINC 2020: Profusa Lumee oxygen platform may improve clinical management of...
Profusa has announced research findings that suggest the company's Lumee oxygen platform may help improve the clinical management of patients with critical limb ischemia...
LINC 2020: Positive new data for AV access interventions released
New arteriovenous (AV) access results were presented at the 2020 Leipzig Interventional Course (LINC; 28–31 January, Leipzig, Germany), including data on percutaneous AV fistula...
Shockwave IVL can aid in eliminating recoil and dissection during below-the-knee...
George Adams (Raleigh, USA) talks to VEITHtv at the VEITHsymposium 2019 (19–23 November, New York, USA) about the benefits of using easy-to-use Shockwave IVL (Intravascular Lithotripsy) in calcified...
MedAlliance receives third FDA breakthrough device designation for its sirolimus DEB...
MedAlliance, the first drug-eluting balloon (DEB) company in the world to receive US Food and Drug Administration (FDA) breakthrough device designation status for a...
CMS approves coverage for PQ Bypass TORUS 2 IDE trial
PQ Bypass announced today that it has received approval for coverage from the Centers for Medicare and Medicaid Services (CMS) for the Investigational Device...
Cardiovascular Systems introduces peripheral orbital atherectomy system with GlideAssist in Europe
Cardiovascular Systems announced today that the first patient in Europe has been treated with its Stealth 360 peripheral orbital atherectomy system (OAS) 1.25mm Micro...
IVUS during haemodialysis access “aids device selection and adequacy of treatment”
Narayan Karunanithy outlines the benefits of intravascular ultrasound (IVUS) during haemodialysis access intervention.
Thoracic central veins include intra-thoracic segments of the internal jugular, subclavian, brachiocephalic...
Reaction to the Katsanos et al meta-analysis
Internal BIBA MedTech data show that perceptions on paclitaxel devices have shifted considerably in the twelve months that have elapsed since Konstantinos Katsanos (Patras,...
More trained interventionalists needed to treat stroke “on the spot”
Nick Hopkins (Buffalo, USA) talks to BLearning Neuro at VEITH Symposium 2018 (13–17 November 2018, New York, USA) about the DAWN trial, which showed that in carefully selected acute...
Benefit and risk: Meta-analysis draws a heterogeneous picture of drug-coated balloon...
Scientists of Jena University Hospital, Germany, conducted a meta-analysis sought to evaluate benefit and risk of paclitaxel-coated balloon angioplasty compared to plain old balloon...
Philips receives FDA approval for low-dose drug-coated balloons
Philips has received US Food and Drug Administration (FDA) approval for two Stellarex 0.035” low-dose (200mm and 150mm) drug-coated balloons for the treatment of...
No evidence of increased mortality with paclitaxel-based devices in German claim...
A real-world safety analysis of paclitaxel-based devices in peripheral arteries, recently published in the European Heart Journal, showed no evidence for increased mortality over...
Medtronic receives FDA breakthrough device designation for its Valiant Navion LSA...
Medtronic today announced it has received breakthrough device designation from the US Food and Drug Administration (FDA) for its Valiant TAAA stent graft system...
Intra-arterial calcification poses “one of the biggest challenges” for endovascular therapy
Andrew Holden (Auckland, New Zealand) provides perspective on the first results from the Disrupt PAD III observational registry that were presented at the Charing...
BD announces publication of drug-coated balloon safety data for femoropopliteal PAD
BD has announced the publication of a company-initiated, independent analysis of the Lutonix 035 drug-coated balloon (DCB) femoropopliteal clinical programme in the Journal of...
TINTIN trial shows “impressive” results at six months
The six-month results of TINTIN (Treatment with the lumINor DCB and The IVolutioN self-expanding stent) trial have been revealed at the annual meeting of the Cardiovascular and...
LimFlow receives FDA approval for US study of minimally-invasive technology designed...
LimFlow has announced that the US Food and Drug Administration (FDA) has approved its investigational device exemption (IDE) for the PROMISE II pivotal study of...
Orchestra BioMed announce FDA breakthrough device designation for Virtue sirolimus-eluting balloon
Orchestra BioMed, in partnership with Terumo Corporation, today announced that the company has secured breakthrough device designation by the US Food and Drug Administration...
FDA clears Artis icono family of angiography systems from Siemens Healthineers
The US Food and Drug Administration (FDA) has cleared the Artis icono, a family of angiography systems from Siemens Healthineers that permit a range...
Proximo Medical named commercialisation partner for Biotronik’s PVI portfolio in select...
According to a press release, Proximo Medical has been announced as the commercial partner for Biotronik's peripheral vascular intervention (PVI) platform in select US markets.
Proximo Medical is...
Novel paclitaxel-nano-coated balloon achieves lower incidence of restenosis than plain balloon...
Two-year results of EffPac—a randomised controlled trial to assess the safety and efficacy of a novel drug-coated balloon (DCB) with a nanotechnology coating—have been...
First patient enrolled in Cook Regentec’s clinical trial of investigational therapy...
Cook Regentec has announced enrolment of the first patient in an international clinical trial evaluating the HemaTrate Blood Filtration System to treat patients with...
New Cook Medical paclitaxel data confirm safety of Zilver PTX
New data on the use of Zilver PTX, Cook Medical’s paclitaxel-coated stent for peripheral arterial disease (PAD), confirms the safety of the device. These...
Beacon Tip catheters available in Europe again
Beacon Tip catheters (Cook Medical) are once again available to physicians in Europe. As of September 2019, the catheters are available in the UK,...
MagicTouch AVF sirolimus-coated balloon granted FDA breakthrough device designation
Concept Medical has been granted breakthrough device designation from the US Food and Drug Administration (FDA) for MagicTouch AVF, its sirolimus drug-coated balloon (DCB)...
Surmodics completes enrolment in pivotal TRANSCEND clinical trial
According to a press release, Surmodics has completed enrolment in TRANSCEND, its pivotal clinical trial for the SurVeil drug-coated balloon (DCB).
“TRANSCEND is a rigorous...
Cardiovascular Systems announces first patient enrolled in REACH PVI clinical study
Cardiovascular Systems recently announced that the first patient has been enrolled in the REACH PVI study. The purpose of this study is to prospectively...
Endologix announces reinstatement of CE mark for its Nellix endovascular aneurysm...
Endologix recently announced that the EC Certificate of Conformity (CE mark) for the Nellix endovascular aneurysm sealing system (Nellix system) has been reinstated by...
Eluvia drug-eluting stent continues to demonstrate positive outcomes in IMPERIAL trial...
Boston Scientific has announced results from sub-analyses of the IMPERIAL clinical trial for the Eluvia Drug-Eluting Vascular Stent System. Data demonstrated that the efficacy...
Canon Medical Systems launches Alphenix interventional imaging line
Canon Medical Systems USA recently introduced its next generation of interventional systems—the Alphenix platform. The new platform of systems incorporates all-new features that enable clinicians...
Gore Tigris vascular stent demonstrates high patency rates at 12 months
New results suggest that the Tigris vascular stent (Gore) is a safe and effective device that can be incorporated into a modern “leave-nothing-behind” treatment...
Vascular Dynamics names Martin Rothman chief medical officer
Martin T Rothman has been appointed as chief medical officer of Vascular Dynamics, effective 1 January 2019.
Rothman is a pioneer in interventional cardiology, as...
BELONG study enrols first patients
Enrolment of the first patients in the BELONG study—benefit of arterial vessel preparation by arterial by longitudinal micro-incisions before drug-eluting balloon angioplasty of the...