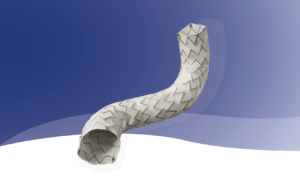
iVascular has received CE mark approval for its balloon-expandable covered stent, iCover, a press release from the company states.
Balloon-expandable covered stents are commonly used for treating arteriosclerotic lesions in renal and iliac arteries, and for the treatment of aneurysms and ruptures.
According to iVascular, iCover can adapt to the most tortuous vessels due to its high flexibility and post-expansion capacity. “It also offers excellent visibility, as it is the only balloon-expandable covered stent with radiopaque markers on the ends of the stent, to facilitate the implantation and the post-expansion,” a company press release details.
iCover is encapsulated with iVascular’s new proprietary technology, CoverTech. This technology attaches the inner and outer expanded polytetrafluoroethylene (ePTFE) layers to ensure a complete encapsulation.
“The first clinical experiences with iCover are remarkable,” states Vicente Riambau (Hospital Clinic of Barcelona, Barcelona, Spain). He adds that “iCover stands out for its excellent navigability and flexibility, while the radiopaque tantalum markers on its ends provide better visibility under X-rays, which enhances precision during implantation. With the launch of iCover, iVascular offers a complete portfolio to treat all type of peripheral lesions.”












