Tag: PAD
Atherectomy in PAD: Meta-analysis of “extensive” published literature supports use in...
A systematic review and meta-analysis of more than 300 original investigations on atherectomy in peripheral arterial disease (PAD) highlights “overall favourable” clinical outcomes. This...
“We need to look at the totality of evidence”: VIVA panellists...
A focused session at the 2025 Vascular Interventional Advances (VIVA) conference (2–5 November, Las Vegas, USA) homed in on the latest findings from the...
BD enrols first patient in XTRACT registry of Rotarex catheter system...
BD has announced the enrolment of the first patient in the XTRACT registry, a prospective, multicentre, single-arm, postmarket registry study designed to evaluate the...
First patient in UK treated with new laser catheter for PAD
University Hospitals Plymouth (UHP) NHS Trust has become the first centre in the UK to treat patients with new laser catheter technology for vascular...
SUCCESS PTA stratified analysis finds equal benefit for sirolimus DEB above...
Positive 12-month outcomes for the Selution Sustained Limus Release (SLR; Cordis) drug-eluting balloon (DEB) were observed, with consistent haemodynamic, functional and clinical improvements irrespective...
SWEDEPAD re-opens paclitaxel safety discussion, finds drug-coated devices do not improve...
Drug-coated balloons and stents were not associated with reduced risk of amputation or improved quality of life compared with uncoated devices in the SWEDEPAD...
Abbott’s Esprit BTK system receives CE mark
Abbott today announced it has received CE mark approval in Europe for the Esprit BTK everolimus-eluting resorbable scaffold system (Esprit BTK system), a technology...
FDA grants approval for IDE study on Advance Evero 18 everolimus-coated...
The Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an investigational device exemption (IDE) study on the Advance Evero...
Medtronic to distribute FMD’s peripheral guidewires for transradial access in the...
Medtronic today announced it has entered into an exclusive US distribution agreement with Japan-based Future Medical Design Co (FMD) to sell specialty and workhorse...
FastWave Medical secures eighth US patent for IVL portfolio
FastWave Medical has secured its eighth US patent for its electric intravascular lithotripsy (E-IVL) platform, Artero, reflecting the company's continued innovation in developing next-generation...
Transcatheter arterialisation of the deep veins: Comparative analysis probes whether emerging...
“We're living in a new world order” when it comes to “no-option” chronic limb-threatening ischaemia (CLTI) patients, says Anahita Dua (Massachusetts General Hospital, Boston,...
BD to initiate real-world patient registry for Rotarex atherectomy system
BD has announced plans to initiate a patient data registry for the Rotarex atherectomy system to measure real-world outcomes for patients with peripheral arterial...
R3 Vascular announces first patient treated in ELITE-BTK pivotal trial
R3 Vascular recently announced that the first patient in its ELITE-BTK pivotal trial has been treated by Brian DeRubertis (New York-Presbyterian and Weill Cornell...
Awaited first-release data and debates set to address critical challenges in...
“These crucial results will help to shape next steps in research, including ongoing randomised controlled trials comparing bioresorbable scaffolds to angioplasty, and eventually, to...
Cagent Vascular launches Serranator SL-PRO for CLTI and pedal disease
Cagent Vascular has announced the launch of the Serranator SL-PRO percutaneous transluminal angioplasty (PTA) serration balloon catheter for chronic limb-threatening ischaemia (CLTI) and pedal...
Data and device tweaks herald ‘practice-changing’ future for bioabsorbable scaffolds
Pending the results of several trials and “iterative changes” to device design, bioabsorbable scaffolds are set to change the treatment paradigm for lower extremity...
Cagent Vascular initiates the Serranator POINT FORCE observational registry
Cagent Vascular has announced the start of the POINT FORCE registry, a postmarket clinical follow-up study of the Serranator percutaneous transluminal angioplasty (PTA) serration...
Drug-eluting technologies should be ‘de facto standard of care’ for PAD,...
New data from a large, real-world study support the use of drug-eluting devices to reduce amputations, readmissions, and healthcare costs in the treatment of...
R3 Vascular secures WCG IRB approval and CMS category B Medicare...
R3 Vascular today announced that it has received WCG Institutional Review Board (IRB) approval for the ELITE-BTK pivotal trial of its Magnitude drug-eluting next-generation...
Study finds adverse outcomes after decreased use of paclitaxel-coated devices
A recent analysis of over 270,000 Medicare fee-for-service beneficiaries has found an increase in adverse outcomes and death after a US Food and Drug...
Biotronik enrols first patients in BIO-OSCAR FIRST trial
Biotronik announced this week that it has enrolled the first patients in its BIO-OSCAR FIRST trial, a study designed to confirm the safety and...
Sensome announces data from two studies showing clot-sensing guidewire successfully identifies...
Sensome today announced positive results from two studies of its Clotild smart guidewire system demonstrating its ability to successfully identify fresh clot—thrombus rich in...
BIO-OSCAR SOC trial examines standard of care in PAD treatment
Biotronik today announced that it has concluded the BIO-OSCAR SOC study evaluating the baseline against which to measure the Oscar multifunctional catheter in treating complex...
Endologix announces 36-month results of DETOUR2 study
Endologix has announced the final 36-month results from the DETOUR2 study—a prospective, single-arm, international, multicentre clinical evaluation of the novel Detour system for fully...
Bolt Medical announces completion of RESTORE ATK and RESTORE BTK pivotal...
Bolt Medical has announced the completion and results of the RESTORE ATK and RESTORE BTK pivotal clinical trials investigating the company's Bolt intravascular lithotripsy...
Two-year LIFE-BTK data show sustained benefits of drug-eluting resorbable scaffold for...
Presented today, late-breaking data from the second year of the LIFE-BTK clinical trial demonstrate the long-term effectiveness of the US Food and Drug Administration...
US FDA grants R3 Vascular IDE approval for ELITE-BTK pivotal trial...
R3 Vascular today announced that the US Food and Drug Administration (FDA) has granted investigational device exemption (IDE) approval to initiate its ELITE-BTK pivotal...
Vesalio announces clinical study initiative for recently launched pVasc thrombectomy system
Vesalio has announced the initiation of a prospective, single-arm, multicentre study supporting the recently launched pVasc thrombectomy system for non-surgically removing peripheral occlusions. This...
Novel RCT framework set to improve design and reporting of endovascular...
Pilot testing has shown that a new framework—dubbed Endo-STAR—can be used to describe and standardise endovascular interventions for peripheral arterial disease (PAD) within a...
AVS enrols first patient in US pivotal intravascular lithotripsy study
Amplitude Vascular Systems (AVS) recently announced that it has enrolled the first patient in its US pivotal trial for pulsatile intravascular lithotripsy (IVL) therapy....
“Game-changing” six-month SUCCESS PTA results report Selution SLR efficacy
The first report of six-month results from the SUCCESS PTA trial evaluating the Selution SLR (Cordis) drug-eluting balloon has shown that consistent haemodynamic, functional...
Shockwave Medical expands US peripheral IVL portfolio with enhanced catheter
Shockwave Medical, part of Johnson & Johnson MedTech, has announced the full US launch of its Shockwave E8 peripheral intravascular lithotripsy (IVL) catheter, following...
Study: Social support associated with better PAD health outcomes
Patients with peripheral arterial disease (PAD) reporting lower levels of social support experience worse health outcomes, a new Yale-led study finds.
Social support is thought...
New ESC guidelines combine peripheral arterial and aortic diseases for first...
The European Society of Cardiology (ESC) has today published its 2024 guidelines for the management of peripheral arterial and aortic diseases (PAAD), evaluating these...
AVS receives IDE approval from US FDA for pivotal intravascular lithotripsy...
Amplitude Vascular Systems (AVS) announced today that it has received an investigational device exemption (IDE) from the US Food and Drug Administration (FDA) to...
PAD guideline update from ACC, AHA and others centres multispecialty care
New multisociety clinical practice guidelines for the management of lower extremity peripheral arterial disease (PAD) have been published online in the Journal of the...
Ultrafast contrast-enhanced ultrasound for PAD patients shows promise in early feasibility...
Majorie van Helvert (University of Twente, Enschede, The Netherlands) and Michel Reijnen (Rijnstate Hospital, Arnhem, The Netherlands) are currently conducting research on ultrafast contrast-enhanced...
Studies highlight need for tailored treatment options for women with peripheral...
New clinical results highlight the need for inclusive approaches and comprehensive examinations of treatment options for peripheral arterial disease (PAD), including endovascular therapy and...
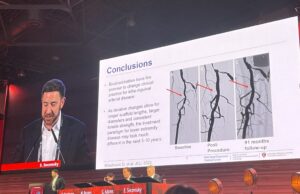
Paclitaxel controversy: Yes, device restrictions did cause harm
“Unfortunately, we are doing worse for our patients today,” were the sobering thoughts of Eric Secemsky (Beth Israel Deaconess Medical Center, Boston, USA) during...
New data and heated debates set to spark controversy in CX...
“These will be important, salutary lessons. Should a controversy arise again involving a proven efficacious therapy, we now know that stopping access to that...
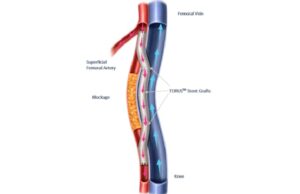
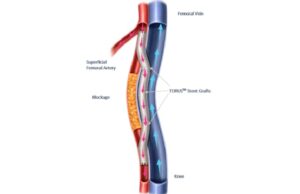
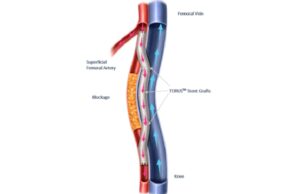
Rotarex™S: A 25-year legacy continues
This advertorial, sponsored by BD, is only available in selected countries and geographies.
This year marks a significant milestone as BD celebrates 25 years...
BD announces first patient enrolled in PAD vascular covered stent trial
BD has announced the enrolment of the first patient in the AGILITY investigational device exemption (IDE) study, which will assess the safety and effectiveness...
Endologix initiates postmarket study of the Detour system
Endologix has announced the initiation of the Percutaneous transmural arterial bypass (PTAB)1 postmarket study. This study marks the beginning of a comprehensive postmarket study...
Cagent Vascular raises US$30 million series C financing
Cagent Vascular has announced a series C financing close in excess of US$30 million. US Venture Partners (USVP) led the round. Participation included new...
IsomAb announces close of £7.5 million financing to accelerate lead candidate...
IsomAb Ltd, a UK-based biotechnology company, has announced the closing of a £7.5 million (approximately US$9.4 million) seed financing round, led by Broadview Ventures, with...
Sensome initiates trial assessing tissue microsensor technology in peripheral artery disease
Sensome has announced enrolment of the first patients into a feasibility clinical study using the Clotild smart guidewire in peripheral artery disease (PAD). Clotild...
‘Get a Pulse on PAD’: Multi-society public awareness campaign launched
The multi-society PAD Pulse Alliance is taking part in a peripheral arterial disease (PAD) public awareness campaign called “Get a Pulse on PAD”.
The group—formed...
UK MHRA update: Paclitaxel-coated device increased mortality risk is withdrawn for...
Following a review, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) has updated guidance on the use of paclitaxel-coated devices stating that such...
AngioDynamics announces US FDA 510(k) clearance of Auryon XL radial access...
AngioDynamics today announced that the US Food and Drug Administration (FDA) has cleared the Auryon XL catheter, a 225cm radial access catheter, for use with...
Summa Therapeutics announce first-in-man injectable angioplasty treatments for PAD
Summa Therapeutics has announced that the first-in-man injectable angioplasty procedures for patients with below-knee peripheral arterial disease (PAD) were performed successfully using its Finesse...
Low-income patients found to receive low-intensity care for CLTI, associated with...
A recent study published in Circulation concerning patients with chronic limb-threatening ischemia (CLTI) in the USA, has found that patients of low-income status and...
US Centers for Medicare & Medicaid Services grants Transitional Pass-Through payment...
Endologix recently announced that the US Centers for Medicare & Medicaid Services (CMS) has granted a Transitional Pass-Through (TPT) payment for the Detour system,...
Interventional News’ top 10 most popular stories of October 2023
Interventional News’ most read stories in October included significant data releases from TCT 2023 (23–26 October, San Francisco, USA); results from a study investigating sex...
Endologix announces results of pooled analysis of DETOUR1 and DETOUR2 Studies...
Endologix has announced results from a pooled analysis of the DETOUR1 and DETOUR2 studies evaluating percutaneous transmural arterial bypass (PTAB) with the Detour system.
A...
LimFlow announces agreement to be acquired by Inari Medical
LimFlow announced today that it has entered into a definitive agreement to be acquired by Inari Medical.
A press release details that, under the terms...
TCT 2023: First presentation of complete patient-level dataset on paclitaxel and...
Data from a patient-level meta-analysis—a factor in the US Food and Drug Administration’s (FDA) recent change of position on the use of paclitaxel-coated devices...
Cardio Flow announces US FDA 510(k) clearance for its FreedomFlow orbital...
Cardio Flow recently announced it has received US Food and Drug Administration (FDA) 510(k) clearance for the company’s FreedomFlow orbital atherectomy peripheral platform.
The company...
US FDA approves LimFlow system for treatment of chronic limb-threatening ischemia
LimFlow, a developer of minimally-invasive technologies for the treatment of chronic limb-threatening ischemia (CLTI), a severe form of peripheral artery disease (PAD), has announced...
Promising future previewed for PAD treatment at Riga summit
This advertorial is sponsored by BD.
There has been major progress in the treatment of peripheral arterial disease (PAD) globally in the last few years,...
FDA update: Paclitaxel-coated devices for PAD cleared of excess mortality risk,...
This advertorial is sponsored by Boston Scientific.
Data on hundreds of thousands of patients who have received lower limb endovascular treatment provide confidence in paclitaxel...
Alucent Biomedical granted US FDA approval for clinical study
Alucent Biomedical has announced that the US Food and Drug Administration (FDA) granted an investigational device exemption (IDE) for a US clinical study of...
Endologix announces first patients treated with the Detour system
Endologix has shared in a press release that the first patients underwent percutaneous transmural arterial bypass (PTAB) using the Detour system since US Food...
Boston Scientific announces position on FDA update about use of paclitaxel-coated...
Following yesterday's news that the US Food and Drug Administration (FDA) has changed its stance on the use of paclitaxel-coated devices to treat peripheral...
Long-awaited US FDA update finds data do not support excess mortality...
In a letter to healthcare providers dated 11 July 2023, the US Food and Drug Administration (FDA) communicates that the risk of mortality associated...
US FDA approves Endologix’s Detour System for treating complex PAD
Endologix has announced in a press release that the US Food and Drug Administration (FDA) has granted approval for the Detour System to treat...
Biotronik launches Oscar multifunctional peripheral catheter at LINC 2023
Biotronik today announced the limited release of its Oscar (One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter and start of promotional activities at this...
New “biopsychosocial” roadmap set out to address PAD treatment and mental...
A recent proposal has called for “whole-person, multidisciplinary interventions” after an interrogation of the interplay between lower extremity peripheral arterial disease (PAD) and mental...
Real-world data consistent with RCTs in highlighting the efficacy of drug-eluting...
This advertorial is sponsored by Boston Scientific.
During a recent satellite symposium, which took place at the 2022 Cardiovascular Interventional Radiological Society of Europe (CIRSE)...
BASIL-2 points towards endovascular-first revascularisation strategy in CLTI patients
A question from Manj Gohel (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) on what the Charing Cross (CX) audience should take back to...
BlackSwan is given FDA premarket approval for new treatment of peripheral...
BlackSwan Vascular, a privately held company that is developing innovative therapies in endovascular embolization, is has announced it has received US Food and Drug...
Getinge receives US FDA premarket approval for the iCast covered stent...
Getinge's iCast covered stent system has received premarket approval from the US Food and Drug Administration (FDA) for the treatment of patients with iliac...
New VOYAGER PAD analysis confirms consistent benefit of low-dose rivaroxaban plus...
Data from a new prespecified analysis of the phase III VOYAGER PAD clinical trial show that low-dose rivaroxaban plus aspirin resulted in a 33%...
Humacyte publish six-year outcomes in study of HAV for peripheral arterial...
Humacyte, a clinical-stage biotechnology platform company developing universally implantable bioengineered human tissues and advanced tissue constructs and organ systems at commercial scale, today announced...
Concept Medical granted IDE approval for Magic Touch sirolimus DCB below...
The Magic Touch percutaneous transluminal angioplasty (PTA) sirolimus drug-coated balloon catheter (DCB) has received investigational device exemption (IDE) for the treatment of below-the-knee (BTK) atherosclerotic...
The BeBack crossing catheter: A “game-changer” in endovascular PAD practice
This advertorial is sponsored by Bentley.
Crossing chronic total occlusion (CTO) lesions are challenging procedures. The BeBack crossing catheter—Bentley’s first product to be available in...
Cardiovascular Systems enrols first patient in Japan for KAIZEN clinical study
Cardiovascular Systems Inc (CSI) recently announced initiation of the KAIZEN clinical study of its Diamondback 360 peripheral orbital atherectomy system (OAS) for the treatment...
“Impressive” 12-month Flex vessel prep data presented at VEITHsymposium
VentureMed Group, a privately held medical device innovator in access management for arteriovenous (AV) fistulas and grafts and vessel preparation for interventional treatment of...
Efemoral Medical closes financing round to fund ongoing study of its...
Efemoral Medical has today announced the closing of its US$4.9 million preferred Series A1 round. Supported by existing investors as well as an experienced...
VentureMed to present new data on DCB treatment for PAD
VentureMed Group has announced new data presentations on the treatment of peripheral arterial disease (PAD) with drug-coated balloon (DCBs) in addition to 12-month AV...
Chocolate Touch drug-coated angioplasty balloon for treatment of peripheral artery disease...
Genesis MedTech Group announced that the US Food and Drug Administration (FDA) has approved the Chocolate Touch drug-coated balloon (DCB) percutaneous transluminal angioplasty (PTA)...
SIRONA trial enrolment completed
Concept Medical has announced the successful completion of enrolment for the SIRONA randomised controlled trial (RCT) investigating the use of its Magic Touch PTA...
Interventional News’ top 10 most popular items of September 2022
Interventional News’ most popular content last month included news of the acquisition of a new catheter by Bentley, the announcement of the final patient...
Terumo announces study results underscoring safety and efficacy of radial to...
Terumo Medical Corporation has announced late-breaking data showing the safety and efficacy of radial to peripheral (R2P) interventions with the R2P Misago self-expanding peripheral...
Truveta announces collaboration with Boston Scientific to advance post-procedure patient insights...
Truveta announced a strategic collaborative agreement with Boston Scientific to improve long-term patient care and gain insights into healthcare disparities. Through this collaboration, Boston...
Merit Medical launches the Prelude Roadster guide sheath
Merit Medical Systems has announced the US commercial release of the Prelude Roadster guide sheath. The Prelude Roadster is the newest addition to the...
Interventional News’ top 10 most read items of August 2022
Interventional News' popular stories last month included acquisitions by Boston Scientific and Gore, studies of deep vein thrombosis and peripheral arterial disease treatments and,...
38th CIRSE annual congress begins this weekend
The European Society of Cardiovascular and Interventional Radiology (CIRSE) will host its 38th annual meeting in Barcelona from September 10 until September 14. Around...
First US patient enrolled in Selution SLR IDE peripheral study
The first US patient has been enrolled in the US Food and Drug Administration (FDA) SELUTION4BTK (below-the-knee) clinical trial evaluating Selution SLR, MedAlliance's novel...
BD launches first-in-human trial of a peripheral sirolimus drug-coated balloon
BD has announced the start of enrolment in a first-in-human trial of a peripheral sirolimus drug-coated balloon (DCB).
The PREVISION trial is a prospective, multicentre,...
Peripheral vascular interventions for PAD: “Confronting the healthcare disparities”
In a study recently published in Circulation, researchers found that Black adults underwent significantly more endovascular peripheral vascular interventions (PVI), were treated for more advanced disease...
AngioDynamics announces FDA clearance of expanded indications for Auryon atherectomy system
AngioDynamics recently announced that it has received US Food and Drug Administration (FDA) 510(k) clearance of an expanded indication for the Auryon atherectomy system...
Selution SLR receives second FDA IDE approval
Selution SLR, MedAlliance's sirolimus-eluting balloon, has received conditional US Food and Drug Administration (FDA) investigational device exemption (IDE) approval to initiate its pivotal clinical...
First patients enrolled in LIMES randomised trial of Magic Touch sirolimus-coated...
Concept Medical has announced the initiation of the LIMES trial, which has enrolled 15 patients to evaluate the safety and efficacy of the company's...
Cordis announces start of enrolment in RADIANCY clinical study in Europe
Cordis has announced the start of the RADIANCY premarket clinical study in Europe.
A press release details that RADIANCY is a prospective, multicentre, single-arm...
New data on Biotronik’s Passeo-18 Lux DCB presented at LINC 2022
Biotronik announced the presentation of two studies on the performance of its drug-coated balloon (DCB) catheter Passeo-18 Lux at the Leipzig Interventional Course (LINC)...
New project set to develop nanoparticles with imaging and medicine-delivery capabilities...
A University of Texas at Arlington (UTA; Arlington, USA) bioengineer is leading a project that will develop biodegradable nanomaterials that will take pictures and...
The vascular biology behind endovascular therapies
Peripheral arterial disease (PAD) produces a global health burden and remains a leading cause of disability, limb loss and reduced quality of life. PAD...
Shockwave IVL maintains superiority to angioplasty in calcified peripheral disease at...
Shockwave Medical announced today that long-term data from the Disrupt PAD III trial found that superior vessel preparation with intravascular lithotripsy (IVL) led to...
Pounce thrombectomy system first-in-human data show 100% technical success in early...
Surmodics has announced that its Pounce thrombectomy system achieved 100% technical success in 20 first-in-human (FIH) procedures. The FIH data were presented by Gary...
Data from new VOYAGER PAD reinforce benefit of XARELTO (rivaroxaban) plus...
An analysis showed benefit of XARELTO plus aspirin in reducing thrombotic hospitalisations for peripheral arterial disease (PAD) patients with and without chronic kidney disease...
New Janssen initiative aims to advance equitable care and address hidden...
Janssen has announced the launch of 'Save Legs. Change Lives. Spot Peripheral Artery Disease Now', a multi-year initiative aimed at creating urgency and action...
Ra Medical Systems reaches 100 enrolled subjects in pivotal atherectomy clinical...
Ra Medical Systems has announced the achievement of a milestone with the enrollment of 100 subjects in the pivotal clinical study, according to a...
Peripheral arterial disease symptoms differ between sexes, meta-analysis finds
In future peripheral arterial disease (PAD) research, clinicians should not consider men and women as a single population and should instead report their data...
PROMISE II U.S. pivotal trial of device designed for ‘no-option’ CLTI...
Enrolment has been completed in the PROMISE II pivotal trial of the LimFlow deep vein arterialisation system designed to prevent amputations in so-called "no-option"...
Ra Medical Systems provides update on enrolment in pivotal atherectomy clinical...
Ra Medical Systems a medical device company focused on developing the excimer laser system to treat vascular diseases, has announced that enrollment has reached...
SIRONA head-to-head randomised trial achieves 50% enrolment
Concept Medical recently announced that the SIRONA randomised controlled trial (RCT; Head-to-head comparison of sirolimus versus paclitaxel drug-eluting balloon angioplasty in the femoropopliteal artery)...
Humacyte’s Human Acellular Vessel for limb salvage evaluated in multiple complex...
Humacyte today announced results from the first series of compassionate use cases of the company’s investigational Human Acellular Vessel (HAV) for the treatment of...
MedAlliance acquires Japanese partner MDK Medical
MedAlliance has acquired its partner MDK Medical, a Japanese vascular specialist company. MDK Medical has previously worked closely with MedAlliance in developing Japanese clinical...
Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee...
VOYAGER PAD subanalysis highlights risks for claudicants undergoing revascularisation
Principal investigator of the VOYAGER PAD trial, Marc Bonaca (University of Colorado Anschutz Medical Campus, Aurora, USA) speaks to Vascular News about the latest data...
Interventional News’ top 10 most popular stories of October 2021
October’s top 10 features an interview with Maureen Kohi (Chapel Hill, USA), the chair and professor of Radiology (University of North Carolina), and the...
Endologix names Matthew Thompson president and CEO
Endologix has announced the appointment of Matthew Thompson as president and chief executive officer. Thompson will also join Endologix’s board of directors. Richard Mott,...
Hispanic adults with PAD access inpatient care most often via the...
Hispanic adults hospitalised for treatment of symptoms of peripheral arterial disease (PAD) were more likely to access this care by going to the emergency...
Head-to-head trial shows superiority of FemoSeal over ProGlide for peripheral interventions
Results of the randomised, prospective, multicentre STEP trial show that the FemoSeal vascular closure system (Terumo) is superior to the Perclose ProGlide suture-mediated closure...
EMINENT one-year results show “superior” primary patency rate for Eluvia DES...
One-year results presented at Vascular Interventional Advances (VIVA) 2021 (5–7 October, Las Vegas, USA) from the EMINENT trial demonstrated the superiority of the Eluvia...
VIVA 2021: Consensus established for appropriate use of IVUS in peripheral...
A worldwide committee of 40 cross-speciality medical experts achieved the first-ever consensus for the appropriate use of intravascular ultrasound (IVUS) in peripheral vascular disease...
VIVA 2021: IVL “consistently” treats real-world calcium in multiple peripheral vessel...
An interim analysis from the DISRUPT PAD III observational study showed that intravascular lithotripsy (IVL; Shockwave Medical) performs “consistently well” across challenging peripheral vessels,...
Two-year Ranger DCB data demonstrate “sustained, high rate” of device efficacy
Patients treated with the Ranger drug-coated balloon (DCB; Boston Scientific) sustained “improved primary patency” with fewer reinterventions than those treated with uncoated devices, two-year...
Target lesion revascularisation in the firing line
Is target lesion revascularisation (TLR) a meaningful clinical endpoint for research or should it be “tossed as a legitimate endpoint for peripheral vascular disease...
First patient treated with Cardiovascular Systems’ ViperCross peripheral support catheter
Cardiovascular Systems recently announced that the first patient has been successfully treated with its ViperCross peripheral support catheter.
Billy J Kim (The Surgical Clinic, Nashville,...
First patient enrolled in BIONETIC-I study of iliac artery treatment with...
Biotronik has announced the first patient enrolment in the BIONETIC-I study of the safety and efficacy of the Dynetic-35 cobalt chromium balloon-expandable stent system...
Japanese Selution SLR study completes enrolment
MedAlliance, with its Japanese partner MDK Medical, has completed enrolment in the clinical study of its novel sirolimus drug-eluting balloon (DEB), Selution SLR, for...
Cardiovascular Coalition urges Congress, CMS to reverse proposed Medicare cuts during...
The CardioVascular Coalition (CVC), a coalition of physicians, care providers, advocates, and manufacturers working to improve awareness and prevention of peripheral arterial disease (PAD),...
Amputation rates higher for people with PAD who are poor or...
Poverty and Black race were associated with higher rates of lower leg amputation among people with peripheral arterial disease (PAD) who live in metropolitan areas, according...
FDA approves expanded PAD indication for Xarelto plus aspirin to include...
The Janssen Pharmaceutical Companies of Johnson & Johnson today announced that the US Food and Drug Administration (FDA) has approved an expanded peripheral arterial...
VOYAGER PAD: Rivaroxaban plus aspirin should be considered after lower extremity...
“A strategy of adding rivaroxaban 2.5mg twice daily to aspirin should be considered after lower extremity bypass regardless of conduit type,” concluded Nicholas Govsyeyev...
Research letter highlights low adoption of supervised exercise therapy for PAD
A research letter published in Circulation: Cardiovascular Quality and Outcomes highlights a “very low” utilisation of supervised exercise therapy (SET) in symptomatic peripheral arterial...
AHA scientific statement provides update on epidemiology, diagnosis, and management of...
The American Heart Association (AHA) has released a new scientific statement on lower extremity peripheral arterial disease (PAD), focusing on contemporary epidemiology, management gaps,...
Researchers recommend “timely interventions by specialists and guideline-based treatment” to reduce...
A population-based study from 2013 to 2015 in Germany has found that nearly one fifth of patients with peripheral arterial disease (PAD) did not...
Nationwide study identifies sex disparities in long-term mortality after paclitaxel exposure
A German claims-based cohort study has revealed that—in 13,204 patients treated with a paclitaxel-coated device for peripheral arterial disease (PAD)—mortality differences were mostly attributable...
XO Score scoring sheath platform wins medical design award
Transit Scientific today announced that its XO Score scoring sheath platform has been named a winner in the 2021 Medical Design Excellence Awards (MDEA)....
Concept Medical releases status updates on SIRONA RCT
Concept Medical has released a series of status updates on their head-to-head SIRONA (Sirolimus versus paclitaxel drug-eluting balloon angioplasty in femoropopliteal diseases) randomised controlled...
Micro Medical Solutions receives FDA breakthrough device designation for MicroStent vascular...
Micro Medical Solutions (MMS) recently announced that the US Food and Drug Administration (FDA) has granted breakthrough device designation for its MicroStent vascular stent....
Introduction of dedicated vascular limb salvage clinic improves one-year amputation outcomes...
One-year outcomes of patients with chronic-limb threatening ischaemia (CLTI) treated in an outpatient-based vascular limb salvage clinic show an improved rate of major amputation....
Acute kidney injury is associated with medium-term mortality following endovascular intervention...
Acute kidney injury is a common complication after intervention for peripheral arterial disease (PAD), and is associated with “medium-term mortality” following percutaneous transluminal angioplasty...
CONSEQUENT study shows low mortality and TLR rates in PAD patients...
NOTE: This video is ONLY available to watch in selected countries and geographies
Ralf Langhoff (Berlin, Germany) talks to Vascular News about the 12-month...
Soundbite announces Health Canada approval for SoundBite crossing system – Peripheral...
Soundbite Medical Solutions has announced Health Canada approval for the SoundBite Crossing System – Peripheral (SCS-P) with the 0.014” Active Wire (14P).
The SCS-P (14P)...
LINC 2021: PRELUDE-BTK results confirm Serranator device novel mechanism of action
Cagent Vascular announced the results of its PRELUDE-BTK study at LINC 2021 (25–29 January, online). The PRELUDE-BTK study was a prospective, single-arm, multicentre feasibility...
Walking can relieve leg pain in people with peripheral arterial disease
Exercise can play an important role in treating peripheral arterial disease (PAD), a recent review from Amy Harwood (Centre for Sport and Exercise Life...
Infinity angioplasty balloon catheter completes first-in-human clinical trial
The Infinity Angioplasty Balloon Company announced today that its angioplasty balloon platform—the Infinity angioplasty balloon catheter—has successfully completed a first-in-human clinical trial.
The platform was...
RCT investigating use of MagicTouch PTA sirolimus-coated balloon for PAD enrols...
Concept Medical has announced the enrolment of the first patient in the SirPAD (Sirolimus in peripheral arterial disease) trial. According to a press release,...
Boston Scientific receives FDA approval for the Ranger DCB
Boston Scientific announced it has received US Food and Drug Administration (FDA) approval of the Ranger drug-coated balloon (DCB), developed for the treatment of...
Ra Medical Systems announces 10 subjects have been enrolled in its...
Ra Medical Systems has announced enrolment of the tenth subject in its pivotal clinical trial to evaluate the safety and effectiveness of the Dabra...
CIRSE 2020: New clinical data support use of 4F devices for...
Outcomes of the BIO4AMB trial demonstrated that ambulatory treatment with 4-French (4F) devices is a valid and safe option for endovascular treatment of lower...
First patient enrolled in FUTURE SFA study of MagicTouch sirolimus-coated balloon
Concept Medical has announced the enrolment of the first patient in the FUTURE SFA (Randomised controlled trial of first sirolimus coated balloon versus standard...
CMS grants additional reimbursement for the Eluvia drug-eluting vascular stent system
Boston Scientific announced that the US Centers for Medicare and Medicaid Services (CMS) granted a new technology add-on payment (NTAP) for the Eluvia drug-eluting...
PQ Bypass receives FDA breakthrough device designation for the Detour system
PQ Bypass has announced that they received breakthrough device designation from the US Food and Drug Administration (FDA) for the Detour system. The Detour...
Philips to expand its image-guided therapy portfolio through acquisition of Intact...
Royal Philips today announced that it has signed an agreement to acquire Intact Vascular. According to a press release, Intact Vascular will enhance Philips’...
High level of heterogeneity found in randomised PAD trial antithrombotic regimens
A recent systematic review showed that randomised trials comparing different types of peripheral endovascular arterial intervention have a high level of heterogeneity in their...
First patient enrolled in PATHFINDER I registry examining Auryon atherectomy system
AngioDynamics has announced the enrolment of the first patient in the PATHFINDER I postmarket registry.
The PATHFINDER I registry is a pilot study to evaluate...
PRIZER, new multicentre PAD study, launches
A new post-market study investigating the effectiveness of the Renzan peripheral stent system (Terumo) in patients with peripheral arterial disease (PAD) has been initiated....
MHRA: Warning to be added to paclitaxel device IFUs in Europe
In a new field safety notice, the UK Medicines and Healthcare products Regulatory Agency (MHRA) states that a warning and clinical summary section will...
SCAI issues device selection guidelines for aortoiliac arterial interventions
The Society for Cardiovascular Angiography and Interventions (SCAI) has released guidelines that provide a comprehensive review of comparative effectiveness data for devices used in...
Wingman catheter granted expanded indication to cross CTOs in PAD
Reflow Medical has announced that they have received US Food and Drug Administration (FDA) clearance for an expanded indication for the Wingman crossing catheter...
Reflow Medical wins FDA breakthrough nod for Temporary Spur stent system
Reflow Medical has announced that its Temporary Spur stent system for treating below-the-knee peripheral arterial disease (PAD) won US Food and Drug Administration (FDA)...
CMS approves coverage for PQ Bypass TORUS 2 IDE trial
PQ Bypass announced today that it has received approval for coverage from the Centers for Medicare and Medicaid Services (CMS) for the Investigational Device...
CIRSE 2019: Hear the latest from clinical trials comparing paclitaxel technologies...
NOTE: ONLY intended for healthcare professionals outside of the USA and France.
Zoom in on a tête-à-tete between Marianne Brodmann (Graz, Austria) and Giovanni Torsello...
New study points to SFDI as promising technology for assessing patients...
New evidence supports the utility of spatial frequency domain imaging (SFDI) for identifying compromised circulation in patients at risk of peripheral arterial disease (PAD)....
CX 2019: Evidence supports safety of paclitaxel-coated devices
At the Charing Cross International Symposium, Gary Ansel (Columbus, Ohio) moderates a global panel that includes Thomas Albrecht (Berlin, Germany), Peter Schneider (San Francisco,...
Depression and peripheral arterial disease: A call to action
With “an indisputable association between depression and peripheral arterial disease (PAD)”—nearly a third of PAD patients experience comorbid depression or depressive symptoms—Joel Ramirez and...
Customising therapy in femoropopliteal revascularisation
Gary Ansel (Columbus, USA) tells Vascular News at VEITHSymposium 2018 about how drug-based therapies have become a mainstay in femoropopliteal revascularisation and how interventionalists...
Smoking cigarettes associated with increased risk of peripheral arterial disease in...
African Americans who smoke cigarettes are more likely than those who do not smoke to develop peripheral arterial disease (PAD), according to new research...
PCSK9 inhibitors, rivaroxaban and a diabetes drug are major milestones in...
Michael Jaff (Newton, USA) outlines the major recent developments in best medical therapy that will impact the treatment of peripheral arterial disease (PAD) at...
Similar patency outcomes in women and men treated with Stellarex DCB
A study presented at VIVA 2018 (5–8 November, Las Vegas, USA) by Maureen Kohi, associate professor of Clinical Radiology, University of California, San Francisco,...
IN.PACT SFA Trial five-year data and Total IN.PACT All-Subjects analysis presented...
The five-year and final results from the pivotal IN.PACT SFA Trial and one-year all-subjects results from the Total IN.PACT Pooled Analysis of the IN.PACT...
Eximo Medical receives FDA clearance for B-Laser atherectomy system to treat...
Eximo Medical has announced it has received 510(k) clearance from the US Food & Drug Administration (FDA) for its B-Laser atherectomy system for peripheral...
Consider intravascular lithotripsy when treating calcified femoropopliteal arteries
Treating severely calcified arteries by endovascular means is still a challenge, although atherectomy, stents and lithotripsy are all techniques that are employed. Lithotripsy (also...
First-ever CE mark of a bioresorbable scaffold for below-the-knee PAD
Reva Medical, a company developing bioresorbable polymer technologies for vascular applications, has announced that its Motiv bioresorbable scaffold is the first drug-eluting bioresorbable scaffold...
Ra Medical files statement for proposed initial public offering
Ra Medical Systems has filed a registration statement on Form S-1 with the US Securities and Exchange Commission relating to a proposed initial public...
Terumo gets CE mark for Kanshas drug-coated balloon catheter for lower...
Terumo has announced receiving the CE mark for its Kanshas drug-coated balloon catheter used in the treatment of lower extremity peripheral arterial disease.
With...
Medtronic receives US FDA clearance for 200mm and 250mm IN.PACT Admiral...
Medtronic has announced that it has received US FDA approval for 200mm and 250mm lengths of the IN.PACT Admiral drug-coated balloon (DCB) to treat...
First patients enrolled in Wing-IT investigational device exemption clinical trial
Reflow Medical announced that the first patients have been enrolled in a prospective, multicentre, non-randomised study intended to evaluate the ability of the Reflow...
US FDA clears CorPath GRX system for use in peripheral vascular...
Corindus has announced receiving 510(k) clearance from the US Food and Drug Administration (FDA) for use of the CorPath GRX system in peripheral vascular...
Cook Medical announces major realignment of its business
Cook Medical has announced key changes that simplify its organisational structure to better support customers.
These changes realign the current sales, marketing, research and...
LimFlow completes enrolment in US feasibility study
LimFlow has announced completion of enrolment of the original 10-patient cohort in the US feasibility study of the LimFlow percutaneous deep vein arterialisation system....
LINC 2018 data highlight IN.PACT Admiral as safe and effective treatment...
Medtronic added to its robust body of clinical evidence supporting the IN.PACT Admiral drug-coated balloon with new presentations that demonstrated durable and consistent clinical...
XableCath gets US FDA clearance for broadly effective catheter aimed at...
XableCath has announced its XableCath blunt tip support catheter has received clearance from the US FDA. The blunt tip catheter facilitates true lumen passage...
PQ Bypass receives IDE approval to initiate pivotal DETOUR II clinical...
PQ Bypass has received conditional approval of its investigational device exemption (IDE) from the US Food and Drug Administration (FDA) to initiate the pivotal...
More than one in six patients with PAD who undergo revascularisation...
A study of nearly 62,000 hospitalisations nationwide in the USA has found that more than one in six patients with peripheral arterial disease (PAD)...
Positive 24-month TOBA results presented at VEITHsymposium
Intact Vascular has announced that positive single centre twenty-four month results from its TOBA (Tack optimised balloon angioplasty) clinical study were presented at VEITHsymposium...
BTG acquires Roxwood Medical
BTG today announces it has acquired Roxwood Medical, an innovative provider of advanced cardiovascular specialty catheters used in the treatment of patients with severe...
Luminor DCB shows high clinical effectiveness in six-month EffPAC data
The six-month results from the full clinical cohort of the EffPAC randomised study were presented in the drug-coated balloon (DCB) abstracts session at the...
Lutonix registry data confirm durability of drug-coated balloon treatment in superficial...
The 24-month results of the worldwide experience with Lutonix 035 drug-coated balloon (CR Bard/Beckton Dickenson) were published online in August in JACC: Cardiovascular Interventions....
Medtronic gets option to purchase QT Vascular’s non-drug coated Chocolate PTA
QT Vascular has announced that it has entered into an asset purchase option agreement with Medtronic, for the acquisition of the non-drug coated Chocolate...
Interrupting inflammatory signals decreases repeat artery blockage in peripheral arterial disease...
Peripheral artery disease patients who were treated with an anti-inflammatory steroid injected directly into the tissue surrounding their leg artery showed a significant reduction...
First US patient treated in vascular study of Lithoplasty technology
PinnacleHealth CardioVascular Institute has enrolled the first US patient in a trial assessing the safety and effectiveness of a new type of approach for...
Barry Katzen announces new global society for critical limb ischaemia
Barry Katzen, Miami, USA, announced the establishment of a new society, the Critical Limb Ischaemia (CLI) Global Society, whose mission is to improve quality...
DANCE data boost for dexamethasone use after revascularisation in femoropopliteal arteries
While drug-eluting devices, be they balloons or stents, use chemotherapeutic agents (usually paclitaxel) to reduce the build-up of scar tissue inside the arteries, a...
US FDA approves first balloon expandable stent graft to treat iliac...
Gore has announced that the Gore Viabahn VBX balloon expandable endoprosthesis has received US FDA approval for treatment of de novo or restenotic lesions...
TVA Medical presents positive everlinQ endoAVF system results at LINC 2017
TVA Medical has provided clinical updates involving its everlinQ endoAVF system at the 2017 Leipzig Interventional Course (LINC; 24–17 January, Leipzig, Germany).
Scientific data were...
Spectranetics gets CE mark for Stellarex 0.014” drug-coated balloon
Spectranetics has announced that its Stellarex 0.014” drug-coated angioplasty balloon has received the CE mark. The device is designed to treat small vessels, below-the...
Lumee Oxygen Platform for continuous, real-time monitoring of tissue oxygen receives...
Profusa has announced that receiving the CE mark to market its Lumee Oxygen Platform for continuous, real-time monitoring of tissue oxygen.
The company will initially...
Shockwave announces positive six-month DISRUPT PAD results in patients with calcified...
Shockwave Medical has announced positive clinical results from the pooled DISRUPT PAD study, a single-arm, two-phase, multicentre study evaluating the safety and performance of...