While drug-eluting devices, be they balloons or stents, use chemotherapeutic agents (usually paclitaxel) to reduce the build-up of scar tissue inside the arteries, a microinfusion device that applies the anti-inflammatory agent, dexamethasone, to the outside of arteries to enhance healing, is seeing largescale early positive results.
Clinical trial data from the DANCE (Delivery of dexamethasone to the adventitia to enhance clinical efficacy after femoropopliteal revascularization) trial that uses the Bullfrog microinfusion device (Mercator MedSystems) were recently presented at two conferences.
Mahmood Razavi and George Adams, the national co-principal investigators of the DANCE trial, each presented comprehensive 13-month data from the trial during late-breaking sessions at the International Symposium on Endovascular Therapy (ISET; 4–8 February, Hollywood, Florida) and Leipzig Interventional Course (LINC; 24-27 January, Leipzig, Germany) respectively.
The Bullfrog microinfusion device was used in the trial to deliver a generic anti-inflammatory steroid, dexamethasone, along with radio-opaque contrast to visualise distribution. The drug was delivered to the tissues around femoropopliteal arteries after undergoing endovascular intervention which forcibly opened obstructions. The data demonstrate a high level of durability of the revascularisation, low occurrence of major adverse limb events, and no device or drug-related adverse events.
“We attribute the success seen in the DANCE trial to the Bullfrog device and its ability to efficiently deliver drug in difficult-to-reach anatomy,” said Razavi. “Technologies such as drug-coated balloons or drug-eluting stents use chemotherapeutic agents to reduce the risk of scar tissue build-up inside the arteries. Alternatively, we have proposed that the delivery of an anti-inflammatory agent to the outside of the artery enhances the healing process and interrupts the cascade leading to scar formation and restenosis. Overall, we have found the therapy in DANCE to be intuitive, safe and effective.”
The 13-month data from DANCE were separated into two groups based on method of revasculariaation: atherectomy was performed in 157 limbs, while 124 limbs received angioplasty without atherectomy. For both groups, the primary safety endpoint revealed no post-operative death within 30 days, with major adverse limb events prior to a revascularisation reported as 1.6% in the atherectomy group and 0.9% in the angioplasty group. The per-protocol primary efficacy endpoint of primary patency did not widely vary between groups, with 83.6% at 12 months and 80.0% at 13 months in the atherectomy group, and 80.2% at 12 months and 78.2% at 13 months in the angioplasty group.
Primary patency was defined as the lack of clinically-driven target lesion revascularisation (CD-TLR) or narrowing detected by a duplex ultrasound peak systolic velocity ratio (PSVR) greater than 2.4. Freedom from CD-TLR was 89.6% at 12 months and 88.7% at 13 months in the atherectomy group and was 89.1% at both 12 and 13 months in the angioplasty group. No broad disparities were reported among results by gender, with 13-month primary patency of 79.3% in men and 77.9% in women, or by diabetic status, with 13-month primary patency of 79.5% in diabetics vs. 78.8% in non-diabetics.
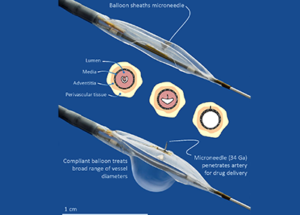
DANCE is a prospective, multicentre, single-arm study designed and sponsored by Mercator. The trial is being conducted in 35 centres. The Mercator Bullfrog is a FDA 510(k)-cleared and CE-marked system designed to infuse therapeutic and diagnostic agents directly, non-systemically and safely through blood vessel walls into adventitial tissues. The Bullfrog device is tipped with a balloon-sheathed microneedle, and is compatible with 0.014” guide wires and 5 to 7 French introducer sheaths. The closed balloon provides a protective covering for a tiny, perpendicular-oriented injection needle as it is guided safely through the vasculature to target vessels with diameters of two to eight millimetres.












