Tag: FDA
FDA begins ‘real-time’ reporting of adverse event data
The Food and Drug Administration (FDA) recently announced that it has begun daily publication of adverse event data from the FDA Adverse Event Reporting...
FDA grants approval for IDE study on Advance Evero 18 everolimus-coated...
The Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an investigational device exemption (IDE) study on the Advance Evero...
US FDA announces plans to address medical device shortage risks
The US Food and Drug Administration (FDA) has issued a statement outlining measures to enhance protections against medical device shortages.
According to Michelle Tarver,...
US FDA issues first draft guidance for AI-based medical device development
The US Food and Drug Administration (FDA) has issued draft guidance that includes recommendations to support development and marketing of safe and effective artificial...
Money-maker or meritable: Is renal denervation out of retirement?
In a paper published in the July 2024 issue of the Journal of Hypertension, Andrew Sharp (University Hospital of Wales and Cardiff University, Cardiff,...
New data and heated debates set to spark controversy in CX...
“These will be important, salutary lessons. Should a controversy arise again involving a proven efficacious therapy, we now know that stopping access to that...
HistoSonics receives FDA clearance for Edison histotripsy system
HistoSonics, the manufacturer of the Edison System and novel histotripsy therapy platforms, has announced the marketing authorisation of its “breakthrough” platform via the...
US FDA seeks to “modernise” clinical trials with new draft guidance
The US Food and Drug Administration (FDA) has released draft guidance with updated recommendations for good clinical practices (GCPs) aimed at modernising the design...
BlackSwan is given FDA premarket approval for new treatment of peripheral...
BlackSwan Vascular, a privately held company that is developing innovative therapies in endovascular embolization, is has announced it has received US Food and Drug...
XACT Robotics receives FDA clearance for its ACE Xtend remote control...
XACT Robotics announced that its ACE Xtend remote control unit received US Food and Drug Administration (FDA) clearance, allowing users to robotically insert and...
Top 10 most popular Interventional News items of July 2022
Prostate conditions and their treatment proved popular reading topics during the month of July. Interventional News reported on this subject from various angles—from an...
Insightec receives FDA IDE approval for prostate cancer comparative study using...
Insightec, a global healthcare company dedicated to using acoustic energy to transform patient care, announced that it has received US Food and Drug Administration...
Ra Medical Systems provides update on enrolment in pivotal atherectomy clinical...
Ra Medical Systems a medical device company focused on developing the excimer laser system to treat vascular diseases, has announced that enrollment has reached...
FDA issues two final guidances for including patient perspectives in medical...
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As...
Medtronic recalls HawkOne directional atherectomy system due to risk of tip...
According to the US Food and Drug Administration (FDA), Medtronic is recalling its HawkOne directional atherectomy system.
The FDA has identified the recall as a...
BD launches Crosser iQ CTO recanalisation system
BD (Becton, Dickinson and Company) has announced the launch and 510(k) clearance from the US Food and Drug Administration (FDA) of the Crosser iQ...
NIH grant sets Mercator research underway for local anti-inflammatory DVT therapy
Mercator MedSystems has recently announced that the DEXTERITY trials research has begun under a technology transfer grant for approximately $300,000 funded by the National...
Interventional News’ top 10 most popular stories of October 2021
October’s top 10 features an interview with Maureen Kohi (Chapel Hill, USA), the chair and professor of Radiology (University of North Carolina), and the...
HistoSonics receives FDA breakthrough device designation for novel sonic beam therapy
HistoSonics has revealed that the US Food and Drug Administration (FDA) has granted the company breakthrough device designation for its new histotripsy targeted liver...
Micro Medical Solutions receives FDA breakthrough device designation for MicroStent vascular...
Micro Medical Solutions (MMS) recently announced that the US Food and Drug Administration (FDA) has granted breakthrough device designation for its MicroStent vascular stent....
FDA grants breakthrough device designation to PEDRA Xauron real-time tissue perfusion...
The US Food and Drug Administration (FDA) has granted Pedra Technology a breakthrough device designation for the periprocedural use of the company’s Pedra Xauron...
CDRH adjusts working practices with medical device companies to deal with...
The US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) has written to the medical device industry to outline its...
LIVE blog: US FDA host public workshop on extended reality in...
The US Food and Drug Administration (FDA) is hosting a public workshop entitled "Medical Extended Reality: Toward Best Evaluation Practices for Virtual and Augmented...
Cerus Endovascular receives FDA approval for its first microcatheter
Cerus Endovascular receives FDA approval for its first microcatheter. The 021 microcatheter will be offered with three different distal configurations, and commercial sales are...
FDA issues guidance on PTA balloon and specialty catheter 510(k) submissions
The US Food and Drug Administration (FDA) has released draft guidance detailing the information device-makers should include in 510(k) submissions of catheter-based devices intended...
With national regulatory approaches diverging, FDA urges “global consensus” at VIVA
Many regulatory jurisdictions are establishing different approaches for medical devices, Misti Malone, assistant director in the Office of Cardiovascular Devices at the US Food...
Medtronic receives FDA breakthrough device designation for its Valiant Navion LSA...
Medtronic today announced it has received breakthrough device designation from the US Food and Drug Administration (FDA) for its Valiant TAAA stent graft system...
LimFlow receives FDA approval for US study of minimally-invasive technology designed...
LimFlow has announced that the US Food and Drug Administration (FDA) has approved its investigational device exemption (IDE) for the PROMISE II pivotal study of...
Orchestra BioMed announce FDA breakthrough device designation for Virtue sirolimus-eluting balloon
Orchestra BioMed, in partnership with Terumo Corporation, today announced that the company has secured breakthrough device designation by the US Food and Drug Administration...
FDA clears Artis icono family of angiography systems from Siemens Healthineers
The US Food and Drug Administration (FDA) has cleared the Artis icono, a family of angiography systems from Siemens Healthineers that permit a range...
Medtronic statement regarding updated FDA letter to healthcare providers on paclitaxel...
Medtronic has issued the following statement regarding the US Food and Drug Administration’s (FDA) updated letter to healthcare providers for paclitaxel-devices in patients with...
FDA: Paclitaxel device clinical trials “may continue”; Agency is working to...
Clinical studies of paclitaxel-coated balloons and paclitaxel-eluting stents “may continue and should collected long-term safety (including mortality) and effectiveness data”, says the US Food...
FDA notifies BD that Lutonix paclitaxel balloon PMA application for below...
The US Food and Drug Administration (FDA) have denied BD premarket approval (PMA) for the company’s Lutonix drug-coated balloon (DCB) in the treatment of...
FDA clears Shape Memory Medical’s Impede-FX embolization plug
The US Food and Drug Administration (FDA) has granted 510(k) clearance for the Impede-FX embolization plug (Shape Memory Medical). This device is available in...
CIRSE updates position on paclitaxel use in peripheral arteries
The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) has released a statement on the use of paclitaxel-coated balloons and stents in the treatment...
Merit’s Embosphere microspheres receive expanded indication to control haemorrhaging in the...
The FDA has cleared an expanded indication of Embosphere microspheres (Merit Medical) for the embolization of blood vessels to occlude blood flow in the...
Scott Gottlieb resigns as head of US FDA
The commissioner of the US Food and Drug Administration (FDA), Scott Gottlieb, has unexpectedly resigned after serving just shy of two years in the...
US FDA grants premarket approval of MANTA vascular closure device
The US Food and Drug Administration (FDA) has given premarket approval (PMA) for the MANTA vascular closure device from Teleflex. This device is the...
US FDA evaluating paclitaxel data, recommend patient surveillance
The impact of the 2018 meta-analysis of randomised paclitaxel-device trials, published in the Journal of the American Heart Association by Konstantinos Katsanos et al,...
US government shutdown disrupting FDA work
The US government shutdown means the country’s Food and Drug Administration (FDA) cannot accept new user fees, which means the agency cannot accept new...
US FDA plan shakeup of its 510(k) clearance programme
The US FDA has announced plans to modernise its 510(k) clearance programme for approving medical devices for the US market. Data show that about...
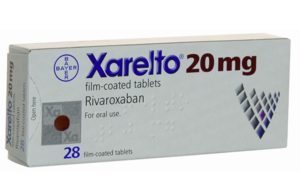
Rivaroxaban receives peripheral arterial disease indication in Europe and USA
Rivaroxaban (brand name Xarelto, Bayer) has received approval from the US Food and Drug Administration (FDA) and the European Commission to reduce risks in...
Valiant Navion thoracic stent graft receives FDA approval
The US Food and Drug Administration (FDA) has approved Valiant Navion thoracic stent graft system (Medtronic) for the minimally invasive repair of all lesions...
Acessa Health wins FDA nod for 3rd generation ProVu
Acessa Health has announced that the company has received US Food and Drug Administration (FDA) 510(k) clearance for its third generation Acessa ProVu radiofrequency ablation...
Cook Medical announces successful resolution of 2014 FDA warning letter
Cook Medical has announced that it received a close-out letter from the US Food and Drug Administration (FDA) resolving a 2014 warning letter for...
Impede embolization plug gets US FDA clearance
Shape Memory Medical announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Impede embolization plug. The device...
Thailand FDA approves new indication for Guerbet’s Lipiodol Ultra-Fluide
Guerbet has announced that the Thailand FDA has given approval of a new indication for Lipiodol Ultra-Fluide for selective hepatic intra-arterial injection for visualisation,...
US FDA grants Medtronic investigational device exemption for new In.Pact Admiral...
Medtronic has received an investigational device exemption (IDE) from the US Food and Drug Administration (FDA) to initiate a study of the In.Pact Admiral...
Teleflex gets US FDA nod for Arrow VPS Rhythm with optional...
Teleflex has announced that its Arrow VPS Rhythm device with optional TipTracker technology has been issued 510(k) clearance so that the device can be...
NICE collaborates with US FDA on Payer Communication Taskforce
NICE’s Scientific Advice programme will work with companies looking to attain both FDA approval and payer coverageThe UK’s National Institute for Health and Care...
Spectranetics gets CE mark for Stellarex 0.014” drug-coated balloon
Spectranetics has announced that its Stellarex 0.014” drug-coated angioplasty balloon has received the CE mark. The device is designed to treat small vessels, below-the...
Medtronic gets FDA clearance of new lower profile HawkOne 6F directional...
Medtronic has received US FDA 510(k) clearance for the HawkOne directional atherectomy system in a new size for treating patients with peripheral artery disease....
Medtronic receives US FDA clearance for TrailBlazer angled peripheral support catheter
Medtronic has announced that the US FDA has cleared the TrailBlazer angled support catheter for use in the peripheral vascular system. Support catheters such...
Cook Medical issues global recall of Roadrunner UniGlide hydrophilic wire guides...
The US Food and Drug Administration has announced that Dutch company DSM Biomedical BV—Cook Medical’s supplier of hydrophilic coating for the Roadrunner Uniglide hydrophilic...