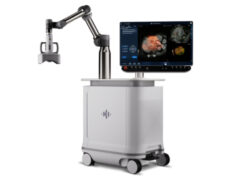
Acessa Health has announced that the company has received US Food and Drug Administration (FDA) 510(k) clearance for its third generation Acessa ProVu radiofrequency ablation system intended for use in treating symptomatic uterine fibroids.
The company, based in Austin, USA, says the newly cleared system features ultrasound visualisation and a guidance mapping system, and that it is intended to provide a safe alternative to hysterectomy procedures for women suffering from uterine fibroids.
“The FDA clearance of Acessa ProVu is a significant step forward in terms of offering patients more minimally invasive options to address their fibroids,” former US Surgeon General Regina Benjamin comments in a press release.
“Acessa Health is delighted to usher in our next phase of innovation with the introduction of Acessa ProVu. Each phase of innovation—the original Acessa system, Acessa guidance system and now the Acessa ProVu system—represent a significant improvement in performance and the opportunity to further support our physician partners who help women suffering from uterine fibroid symptoms,” president and CEO Kim Rodriguez states.
In February, Acessa Health announced that 32 US state Medicaid programs now provide access to the company’s Acessa procedure designed to treat uterine fibroids using radiofrequency ablation.
The FDA initially granted clearance to the first generation Accesa system in 2012, when it was a brand new medical technology used in the treatment of uterine fibroids. The announcement of this first market clearance was during the opening session of the Association of Gynecological Laparoscopists (AAGL) Global Congress on Minimally Invasive Gynecology.