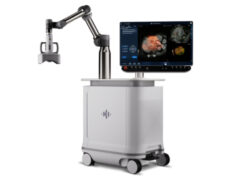
HistoSonics has revealed that the US Food and Drug Administration (FDA) has granted the company breakthrough device designation for its new histotripsy targeted liver therapy platform which is designed to help provide timely access to non-invasive liver treatment.
HistoSonics say that histotripsy of the liver provides clinicians with the first automated external beam therapy using acoustic energy to mechanically destroy and liquefy tissue in the liver without incisions, ionising radiation or heat.
Mike Blue, president and CEO of HistoSonics, sad: “the breakthrough device designation is a significant milestone for our company and validates our belief that our platform offers significant advantages over existing approved or cleared alternatives, per FDA requirements.
“Early and ongoing clinical results are promising and suggest that our ability to precisely destroy targeted liver tissue, completely non-invasively, and without the challenges associated with ionising radiation or other locoregional therapies, provides advantages to patients and physicians that don’t exist today, and we look forward to working with the FDA to make the technology accessible as quickly as possible.”
The company believes the novel mechanism of action of their proprietary technology may offer significant advantages to patients, including equivalent treatment effect throughout the entire treatment volume resulting in precise and predictable treatment zones. Early clinical and pre-clinical results also suggest that histotripsy largely preserves critical structures such as the liver capsule, and larger vessels and ducts within or adjacent to the treated volume of tissue.
In addition, histotripsy gives the treating physicians the ability to monitor the destruction of tissue under continuous real time visualisation and control, unlike any modality that exists today. The Breakthrough Designation will allow the company to engage with the FDA in a prioritised review during the regulatory market authorisation process, the company adds.
HistoSonics has worked with the FDA for over three years in developing pre-clinical and clinical data required for regulatory market authorisation and intends to continue collaborating with the agency throughout the ongoing Investigational Device Exemption (IDE) study, #HOPE4LIVER US, which is designed to evaluate the safety and technical efficacy of histotripsy in patients with primary and secondary liver tumours.
The company plans to share US and European #HOPE4LIVER study data and results with FDA to demonstrate the benefits of histotripsy in a broad patient population.
The HistoSonics system is investigational and is not available for sale in the United States or Europe. It is limited to investigational use in the approved IDE and European studies.