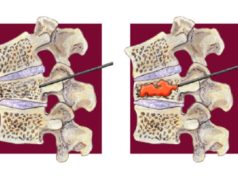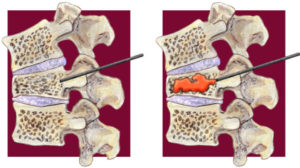
Current evidence does not support the use of vertebroplasty for the treatment of pain from vertebral fractures, concludes a recent task force report charged by the American Society for Bone and Mineral Research (ASBMR) aimed at examining the efficacy and safety of vertebral augmentation. Although lead author of the report, Peter R Ebeling (Monash University, Melbourne, Australia), and colleagues surmise that this conclusion is “likely to apply to other augmentation procedures”, such as kyphoplasty, they maintain throughout the report that high-quality evidence from placebo-controlled trials remains absent. However, lead authors of the VAPOUR trial, which reported a benefit with the use of vertebroplasty in fractures of less than six weeks’ duration, have published their concerns in the BMJ Evidenced-Based Medicine regarding the statistical methodology underpinning the ASMBR report.
“The recommendations by this task force are designed to help provide a foundation for advancing the research and clinical care of patients with painful acute vertebral fractures”, write the authors. They stress that clinicians will need to balance the “limited findings” on the safety and efficacy of other nonpharmacological interventions with good clinical judgement, when it comes to making quality, informed patient care decisions.
The rationale behind the commissioned report encompasses the lack of consensus amongst clinicians on whether to use percutaneous vertebroplasty or kyphoplasty to reduce pain in patients with vertebral fractures. This disparity in opinion, as well as the high prevalence of vertebral compression fractures in patients with osteoporosis, led the ASBMR to commission a task force to address key questions on the efficacy and safety of vertebral augmentation and other nonpharmacological approaches, such as spinal bracing or exercise, for the treatment of pain after vertebral fractures.
Ebeling and colleagues explain that vertebral augmentation was introduced into practice before high-quality evidence establishing its efficacy and safety was published, yet remains—in some settings—part of standard routine care. Although the authors acknowledge that balloon kyphoplasty is currently more expensive and is performed almost three times more commonly than percutaneous vertebroplasty in the USA, they write: “No placebo-controlled trials of balloon kyphoplasty have been performed and the evidence of the value of this procedure is reliant on low-quality evidence from trials that have compared kyphoplasty with usual care or head-to-head comparisons with vertobroplasty”.
Ebeling and colleagues performed a systematic review of the existing literature and meta-analyses of an array of outcomes in order to test the efficacy and safety of vertebral augmentation and other nonpharmacological treatments for painful vertebral fractures. Randomised controlled trials (RCTs) and quasi-randomised trials that enrolled adults older than 40 years with acute nontraumatic vertebral fractures, directly comparing percutaneous vertebroplasty or balloon kyphoplasty with any treatment comparator group, were included. In terms of nonsurgical interventions, Ebeling and colleagues included RCTs and quasi-randomised trials of bracing or exercise interventions with similar enrolment criteria.
The ASBMR task force first addresses the efficacy and relative effectiveness of vertebral augmentation therapies and their ability to improve pain, posture, physical function and quality of life. Based on “moderate to high” quality evidence for percutaneous vertebroplasty from five randomised placebo-controlled trials, Ebeling and colleagues find that percutaneous vertebroplasty provide no demonstrable clinically important benefit when compared with placebo or sham. Moreover, they report that subgroup analyses in the updated Cochrane review indicated that the result did not differ according to duration of pain. However, the sensitivity analyses indicated that open trials comparing percutaneous vertebroplasty with standard medical care are likely to have overestimated any benefit of percutaneous vertebroplasty.
William Clark (St George Private Hospital, Sydney, Australia) and colleagues submitted complaints to the authors of the aforementioned Cochrane review of vertebroplasty, claiming that its conclusions were inaccurate, and advocating for the use and efficacy of this procedure in patients with acute pain of under three weeks’ duration.
In terms of balloon kyphoplasty, Ebeling and colleagues write: “There is insufficient evidence to support kyphoplasty over nonsurgical management, percutaneous vertebroplasty, vertebral body stenting, or KIVA”. Although the authors found that kyphoplasty was associated with improved pain, back-related disability, and quality-of-life outcomes compared with nonsurgical management, they acknowledge that these results were derived almost solely from a single trial. Further, the investigators write: “Any apparent benefits of kyphoplasty over nonsurgical management appeared to decrease over time, and, based on available data, it was not possible to determine whether these between-group differences were clinically meaningful of the extent to which they were accounted for by sham effects or study bias.”
Ebeling and colleagues went on to address the potential harms and possible risk of new vertebral fractures with vertebral augmentation. Prior to the task force report, limited evidence was available on these risks. In relation to both percutaneous vertebroplasty and balloon kyphoplasty, the authors found that adverse events—including risk of death, incident vertebral fracture, cement leakage, adjacent fractures, vasovagal reactions, cord compression requiring decompression, hypoxia and respiratory failure—were rarely systematically reported. According to the authors, compared with nonsurgical management, kyphoplasty was not associated with a statistically significant increased risk of incident vertebral fracture, although it was associated with nearly twice the risk of any adverse event within 30 days of intervention. Yet, Ebeling et al alluded to the high risk of bias present in the trials and they write “it is uncertain whether any benefits of kyphoplasty versus nonsurgical management of vertebral fracture outweigh potentials harms”.
Finally, the efficacy and safety of other nonpharmacologic treatments—spinal bracing and exercise interventions, was investigated. Based on the current evidence, Ebeling and colleagues report that for patients with painful vertebral fractures, there is low quality evidence that spinal bracing improves pain, trunk muscle strength, kyphosis, pulmonary volume and quality of life at six months. Furthermore, although the authors note that, following exercise interventions, the “magnitude of effects on mobility were small”, they surmise that exercise interventions have significant positive effects on mobility and may improve pain, fear of falling, as well as back extensor strength or endurance.
Yet, the aforementioned study biases and methodological flaws present in the literature that Ebeling and colleagues acknowledge throughout the report led them to address these gaps in the literature. The authors report on future research that is required to improve patient outcomes in managing osteoporotic vertebral fractures, in relation to both vertebral augmentation and other nonpharmacologic approaches. While a rigorous trial design to minimise the potential for bias was recommended in order to examine all therapies, specific recommendations were also detailed.
“No further trials of vertebroplasty should be performed, unless they are adequately powered to alter the conclusions of the current body of evidence”, write Ebeling and colleagues. If further trials are to commence, the authors state that participants of these procedures, as well as ethics committees, should be fully informed about the current body of evidence. Regarding balloon kyphoplasty, the authors recommend that “further studies are needed to resolve whether kyphoplasty increases the risk of future vertebral fractures or adverse events, which should be systematically collected”, while “any future trials should carefully characterise the timing and severity of vertebral fracture among study participants [and] should have a placebo control group.”
For spinal bracing and exercise interventions, Ebeling et al maintain that more research is needed in general, including clearly defined participant selection criteria and study protocols. Specifically, more data is needed on serious adverse events, in both the intervention and comparator groups, and on the efficacy of exercise after acute vertebral fracture. Lastly, they note that “future exercise trial designs should account for low adherence and attrition in sample size calculations”.
Based on the findings outlined in the report, the ASBMR task force detailed guidelines for the clinical management of patients with vertebral fracture. Maintaining that the optimal management of vertebral fracture remains uncertain, Ebeling and colleagues put forward that when vertebral augmentation is used, patients should be fully informed about the evidence.
In light of the findings, they conclude: “It is critical that anti-oseoporotic medications are started, continued or changed in patients with recent vertebral fracture”. Further, the task force highlights that while the use of bracing in reducing pain immediately after vertebral fracture was not supported by current evidence, exercise may improve mobility and may reduce pain and fear of falling.
Authors of the VAPOUR trial submit complaint about the Cochrane vertebroplasty review
Interventional radiologist William Clark (St George Private Hospital, Sydney, Austrialia) reached out to Interventional News to say that a section of the ASBMR report takes its findings directly from the Cochrane review. Clark and fellow authors of the VAPOUR trial have submitted a complaint to the chief editor of the Cochrane Library about alleged irregularities in the Cochrane Vertebroplasty Review (CVR) of April 2018. They asked that the review be withdrawn from the Cochrane Library, writing “we believe that the report provides a biased analysis of the VAPOUR trial, contains inappropriate statistical analysis and errors in data presentation.”
CVR authors issued a new November 2018 version of CVR to correct errors in analysis 8.1/2, but according to its critics left the bulk of the review unchanged. Clark and colleagues subsequently published their criticism of the CVR in the BMJ Evidence-Based Medicine. Their allegations against the CVR include bias against the VAPOUR trial, breach of protocol, improper use of meta-analysis and other methodological errors.
While the CVR authors conclude that the benefits from vertebroplasty in the VAPOUR trial are “clinically irrelevant and consistent with the negative results of their trials”, Clark and colleagues write that “this assertion is false”.
Indeed, Clark told Spinal News International that “The same criticisms of the Cochrane review apply directly to the ASBMR report, which draws its conclusions directly from the Cochrane review, which includes the flawed Cochrane meta-analyses.