Tag: Philips
Philips expands commercial availability of LumiGuide imaging system
Philips has announced the expanded commercial availability of LumiGuide 3D device guidance—the much-heralded first real-time artificial intelligence (AI)-enabled light-based navigation solution for image-guided therapy—across...
Philips issues Class I recall for Tack endovascular system
Philips has recently announced that it will no longer sell its Tack endovascular system in the USA following a Class I recall issued by...
First patient enrolled in study of integrated laser atherectomy and IVL...
Royal Philips has announced enrolment of the first patient in the US THOR IDE clinical trial, which will study an innovative combined laser atherectomy...
Philips announces US FDA approval for enhanced LumiGuide guidewire, marks 1000th...
Royal Philips today announced the introduction of the 160cm US Food and Drug Administration (FDA)-approved version of its LumiGuide endovascular navigation wire. This enhanced...
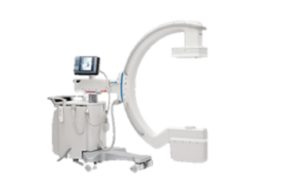
Philips launches new motorised mobile C-arm system
Royal Philips has announced the launch of their image-guided therapy mobile C-arm system 9000—Zenition 90 Motorized, designed to help surgeons deliver high-quality care to...
Duo venous stent system shows 90.2% primary patency at 12 months,...
The Duo venous stent system (Vesper Medical/Philips) showed a 98.7% freedom from major adverse events (MAEs) at 30 days and 90.2% primary patency at...
Philips extends company’s mobile C-arm portfolio with Zenition 10
Philips has announced the launch of Zenition 10, a new addition to the company’s Zenition mobile C-arm series. Based on Philips’ flat panel detector...
Northeast Scientific awarded 510(k) clearance to reprocess the Eagle Eye Platinum...
Northeast Scientific announced this week it has received US Food and Drug Administration (FDA) 510(k) clearance for reprocessing the intravascular ultrasound (IVUS) Eagle Eye...
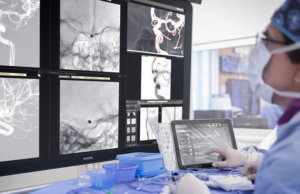
Philips gives updates on spectral detector angio CT solution development
Royal Philips today announced new milestones in the development of the world’s first spectral detector angio computed tomography (CT) solution— Philips Spectral Angio CT...
Safer imaging technology for complex aortic repairs uses light instead of...
A new imaging device at University of Texas (UT) Southwestern (Dallas, USA) is making complex aortic repairs safer for patients and operating room staff...
Vascular experts establish appropriate use of IVUS in peripheral interventions
Royal Philips today announced an important milestone in the evolving standard of care for treating patients with peripheral vascular disease: the establishment of the...
Philips AI-powered, software-defined magnetic resonance software receives 510(k) clearance
Philips has announced that its SmartSpeed artificial intelligence (AI)-powered magnetic resonance (MR) acceleration software has received US Food and Drug Administration (FDA) 510(k) clearance....
Philips announces positive three-year clinical research results from its TOBA II...
Royal Philips today announced the latest results from the Tack optimised balloon angioplasty (TOBA) II below-the-knee (BTK) clinical trial, demonstrating that the Philips endovascular...
Large-scale IVUS analysis adds “meaningful data” to growing pool of evidence
A large-scale analysis of the use of Philips' intravascular ultrasound (IVUS) in lower extremity peripheral vascular interventions adds “meaningful data” to a growing pool...
Philips acquires Vesper Medical
Royal Philips today announced that it has signed an agreement to acquire Vesper Medical, a US-based medical technology company that develops minimally-invasive peripheral vascular...
Philips announces large-scale study outcomes on use of IVUS in peripheral...
Royal Philips today announced the results of a new large-scale real-world analysis of Centers for Medicare & Medicaid Services (CMS) data on the health...
VIVA 2021: Consensus established for appropriate use of IVUS in peripheral...
A worldwide committee of 40 cross-speciality medical experts achieved the first-ever consensus for the appropriate use of intravascular ultrasound (IVUS) in peripheral vascular disease...
Philips Azurion with SmartCT – first user experiences
After a successful introduction of Azurion with SmartCT in 2020, many users have experienced working with the new solution from Philips. Watch the video...
Philips receives FDA Breakthrough Device designation for laser-assisted IVC filter removal...
Royal Philips today announced the US Food and Drug Administration (FDA) has granted Breakthrough Device designation for a laser-assisted inferior vena cava (IVC) filter...
The next leap in simplifying and advancing 3D imaging
This educational supplement is sponsored by Philips.
In this supplement:
Spotlight on the next-generation Azurion: Simplifying 3D imaging with SmartCT
How SmartCT streamlines IR workflow
...
Philips announces positive two-year data from TOBA II BTK clinical trial
Royal Philips recently announced positive two-year results from the TOBA (Tack optimised balloon angioplasty) II below-the-knee (BTK) clinical trial.
The data show the Philips...
Bringing 3D into the lab: SmartCT, the next-generation imaging system
Constantino Peña (Miami, USA) moderates an Interventional News webinar focusing on how the SmartCT system (Philips) can “transform” IR suites and the importance of...
Philips SmartCT application software receives FDA 510(k) clearance
Royal Philips today announced US Food and Drug Administration (FDA) 510(k) clearance for its Philips SmartCT application software. SmartCT is a key component of...
Azurion image guided therapy platform “takes integration to the next level”
Atul Gupta (Philadelphia, USA), chief medical officer of Image Guided Therapy at Philips, comments on the SmartCT clinical application software–part of the Azurion image...
Five-year ILLUMENATE results confirm safety profile of Philips Stellarex DCB
Royal Philips has announced the final, five-year results of two randomised controlled trials (RCTs) that show no difference in all-cause mortality between patients treated...
Philips launches QuickClear mechanical thrombectomy system for blood clot removal in...
Royal Philips has announced the launch of the QuickClear mechanical thrombectomy system. The single-use system delivers an all-in-one aspiration pump and catheter to remove...
The value of IVUS in endovascular procedures
In this supplement:
Paul Gagne explains how intravascular ultrasound (IVUS) can be used to guide the treatment of chronic venous disease, discussing the various...
Win for paclitaxel as SAVER registry “reinforces the safety profile” of...
“The SAVER registry reinforces the safety and effectiveness profile for the Stellarex drug-coated balloon (DCB; Philips) in a real-world patient population,” Konstantinos Stavroulakis (St...
Philips to expand its image-guided therapy portfolio through acquisition of Intact...
Royal Philips today announced that it has signed an agreement to acquire Intact Vascular. According to a press release, Intact Vascular will enhance Philips’...
New data reconfirm long-term safety profile of the Philips Stellarex low-dose...
Royal Philips recently announced the four-year results from the ILLUMENATE European randomised controlled trial (EU RCT). The Stellarex drug-coated balloon (DCB) cohort demonstrated similar...
Philips and the US government collaborate in ventilator production ramp up...
Royal Philips has announced that the US government and Philips agreed to team up to increase the production of hospital ventilators in its manufacturing...
LINC 2020: Four-year ILLUMENATE data reaffirm safety profile of Stellarex
Philips today announced four-year results from the randomised controlled ILLUMENATE pivotal trial in the USA. The data show similar mortality rates through four years...
Philips announces WE-TRUST multicentre clinical trial to assess impact of direct-to-angiosuite...
Philips has announced a major clinical trial to assess the impact of a direct-to-angiosuite workflow on stroke patient outcomes. The study will assess whether...
A glimpse of the future: The next decade of interventional innovations
Interventional News speaks with Atul Gupta about the future of interventional radiology (IR). As a chief medical officer for Image-Guided Therapy, Philips, he enthuses...
Philips receives FDA approval for low-dose drug-coated balloons
Philips has received US Food and Drug Administration (FDA) approval for two Stellarex 0.035” low-dose (200mm and 150mm) drug-coated balloons for the treatment of...
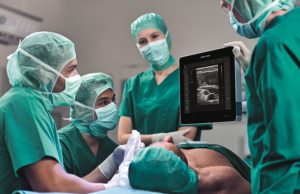
Philips and B Braun launch Onvision needle tip tracking, a breakthrough...
Philips and B Braun have announced the launch of Onvision, a breakthrough ultrasound guidance solution for real-time needle tip tracking in regional anaesthesia. Available...
Late-breaking ILLUMENATE data demonstrate three-year safety and efficacy for Stellarex DCB
Three-year results from the ILLUMENATE Pivotal trial and the ILLUMENATE European randomised controlled trial (EU RCT) have been presented in a late-breaking trial session...
Philips launches new IntraSight interventional applications platform
Philips has announced the launch of its new IntraSight interventional applications platform. According to a press release, the secure, application-based platform offers a comprehensive...
Augmented reality surgical technology unveiled by Philips and Microsoft
At the MWC (25–26 February, Barcelona, Spain), formerly the Mobile World Congress, Philips unveiled a unique mixed reality concept developed in partnership with Microsoft for...
Philips launches Zenition mobile C-arm platform
Philips Zenition, a new mobile C-arm imaging platform from Philips, has launched in the USA, Germany, Austria and Switzerland. It is being introduced into...
New data release at LINC 2019 reinforces safety profile of low-dose...
Sean Lyden (Cleveland, USA) and Fabrizio Fanelli (Rome, Italy) discuss the safety and future of drug-coated balloons (DCB) in light of the latest pooled...
Philips launches Azurion with FlexArm to aid image-guided procedures
Philips launches its Azurion with FlexArm to aid patient imaging and positioning during image-guided procedures.
During increasingly complex interventions, clinicians need to quickly and easily...
Philips launches Azurion with FlexArm to aid image-guided procedures
Philips launches its Azurion with FlexArm platform to aid patient imaging and positioning during image-guided procedures.
Azurion with FlexArm allows clinicians to intraoperatively visualise patient...
First US patient enrolled in ILLUMENATE BTK study of Stellarex 0.014...
The first US patient has been enrolled in the Stellarex ILLUMENATE below-the-knee (BTK) investigational device exemption (IDE) study, led by principal investigators Bill Gray...
Similar patency outcomes in women and men treated with Stellarex DCB
A study presented at VIVA 2018 (5–8 November, Las Vegas, USA) by Maureen Kohi, associate professor of Clinical Radiology, University of California, San Francisco,...
Philips acquires VitalHealth
Royal Philips has announced that it has acquired VitalHealth, a provider of cloud-based population health management solutions for the delivery of personalised care outside of...
New study shows significant clinical workflow and staff experience benefits of...
Philips has announced the results of a comprehensive, independent, two-year study demonstrating the clinical workflow benefits of its next generation image-guided therapy platform, Azurion.
The...
Philips completes acquisition of Spectranetics
Philips has announced that it has completed the acquisition of The Spectranetics Corporation, a US-based global leader in vascular intervention and lead management solutions....
Philips acquires CardioProlific
Royal Philips has announced the acquisition of CardioProlific Inc, a USA-based, privately-held company that is developing catheter-based thrombectomy approaches to treat peripheral vascular disease. Financial...
Philips sets sights on Spectranetics, also acquires CardioProlific
Royal Philips and The Spectranetics Corporation announced that they have entered into a definitive merger agreement. Pursuant to the agreement, Philips will commence a...
The interface of an imaging system should feel “like an extension...
In February, Philips announced the global launch of Azurion, its next generation image-guided therapy platform, which forms the new core of its integrated solutions portfolio....
Pioneer Plus catheter re-entry device with intravascular ultrasound guidance relaunched
Philips has announced the relaunch of the Pioneer Plus catheter, described as the only re-entry device with intravascular ultrasound (IVUS) capabilities and needle deployment...
Philips launches Azurion worldwide
Philips has announced the global launch of Azurion, its next generation image-guided therapy platform, which forms the new core of its integrated solutions portfolio...