Tag: Cook Medical
Cook Medical and Siemens Healthineers announce strategic commercial partnership
Cook Medical and Siemens Healthineers announce a strategic commercial partnership aimed at setting a new standard for interventional medicine.
In a recent press release the...
FDA grants approval for IDE study on Advance Evero 18 everolimus-coated...
The Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an investigational device exemption (IDE) study on the Advance Evero...
Cook Medical issues Class I recall for Beacon Tip angiographic catheters
Cook Medical has initiated a Class I recall of its Beacon Tip 5Fr angiographic catheter.
This follows reports of tip separation that could result...
Real-world data show Cook’s Zilver PTX leads to lower rates of...
Cook Medical has announced that its Zilver PTX drug-eluting stent (DES) has lower rates of in-stent occlusions among patients with restenosis at three years than the Eluvia DES (Boston Scientific), according to...
Cook Medical announce first US patient treated with Zenith Alpha 2...
Cook Medical’s Zenith Alpha 2 (ZTA2) thoracic endovascular graft is now available throughout the United States. A company press release states ZTA2 is the...
Cook Medical announces investment in Zenflow urinary obstruction device
Cook Medical has announced a strategic investment in the urology space via Zenflow, a medical device company developing a minimally invasive treatment for urinary...
Zilver Vena stent shows three-year patency across patient groups
Cook Medical’s Zilver Vena venous self-expanding stent has shown high rates of patency sustained for three years, according to recently published data in the Journal of Vascular...
Cook Medical announces Beacon sizing tip catheter availability in USA and...
Cook Medical have announced that Beacon tip sizing catheters are now available in the USA and Canada. The Beacon catheter is available in a...
Getinge and Cook Medical enter US commercial distribution agreement for iCast...
Getinge and Cook Medical today announced an exclusive sales and distribution agreement for the iCast covered stent system, which recently received US Food and...
Cook Medical announce partnership with catheter securement device company Bedal International
Cook Medical, a global medical device manufacturer, has partnered with Bedal International, a company specialising in catheter securement devices marketed under the brand FlexGRIP....
Cook Medical reports first patient treated in ZFEN+ fenestrated endovascular graft...
Cook Medical has announced the first patient treated in the clinical study of the ZENITH FENESTRATED+ endovascular graft (ZFEN+) in the USA. The procedure was performed...
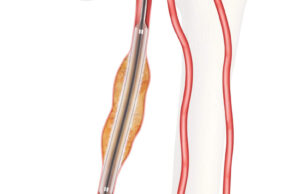
Advance Serenity PTA balloon now available in more sizes and locations
As announced at the Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM 2023; 14–17 June, National Harbor, USA), the Advance Serenity hydrophilic percutaneous...
Study finds fibred embolization coils reduce procedure times and radiation exposure
Following the publication of an animal study examining the performance of embolization coils in arteries in the Journal of Vascular and Interventional Radiology (JVIR)...
Interventional News’ top 10 most popular stories of December 2022
Interventional News’ most popular stories as 2022 came to a close included news of a US Food and Drug Administration clearance for a new...
Cook Medical appoints John A Kaufman as chief medical officer
Cook Medical announces John A Kaufman (Cook Medical, Bloomington, USA) will be joining as its chief medical officer, effective July 2023. With Kaufman’s expertise...
Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee...
Five-year STABLE II results show Zenith system makes the cut for...
Joseph V Lombardi (Cooper University Hospital, Camden, USA) reported long-term outcomes favouring the continued safety and effectiveness of a composite device (proximal covered stent...
FDA grants breakthrough device designation for Zenith fenestrated+ endovascular graft
The Zenith fenestrated+ endovascular graft (ZFEN+) product (Cook Medical) has received breakthrough device designation from the US Food and Drug Administration (FDA). This designation...
New workhorse wire guide in US and Canada now commercially available
The Approach Authority Workhorse microwire guide (Cook Medical) is now commercially available in the USA and Canada.
Designed as an 0.018-inch diameter workhorse wire guide,...
One-year VIVO results support safety and effectiveness of Zilver Vena venous...
Results of the VIVO clinical study support the safety and effectiveness of Cook Medical's recently FDA-cleared Zilver Vena venous stent for the treatment of...
Calls in Germany for reimbursement as analysis shows drug-eluting technology is...
Stefan Müller-Hülsbeck (Ev Luth Diakonissenanstalt zu Flensburg, Flensburg, Germany) presented attendees of the online meeting of the Cardiovascular and Interventional Radiological Society of Europe...
New prediction model for target lesion revascularisation grants “new level of...
“Please feel free to utilise this in your practice,” Michael Dake (University of Arizona Health Sciences, Tucson, USA) urged on the first day of...
Advance Serenity hydrophilic PTA balloon catheter now available in the USA
Cook Medical recently announced that the Advance Serenity hydrophilic percutaneous transluminal angioplasty (PTA) balloon catheter is now available to physicians in the USA. The...
Transluminal biliary biopsy forceps set and Zilver 635 biliary stent now...
The Transluminal Biliary Biopsy Forceps Set (BBFS) and a new 40-centimetre delivery system line extension of the existing Zilver 635 Biliary stent (both from...
New balloon catheter available in USA for use in the treatment...
The Advance Serenity Hydrophilic percutaneous transluminal angioplasty (PTA) balloon catheter is now available to physicians in the USA. The catheter is manufactured by Surmodics...
Cook Medical launches TriForce peripheral crossing set
The TriForce peripheral crossing set, from Cook Medical, is now commercially available in the USA. As of January 2020, these products are available to...
VIVA 2019: Zilver PTX shows benefits across different patient groups
At this year’s Vascular Interventional Advances (VIVA) conference (4–7 November, Las Vegas, USA), Michael D Dake presented data on Zilver PTX that supports the...
Beacon Tip catheters available in Europe again
Beacon Tip catheters (Cook Medical) are once again available to physicians in Europe. As of September 2019, the catheters are available in the UK,...
Cook Medical releases patient-level data from Zilver PTX paclitaxel-coated balloon study
Cook Medical has released de-identifiable patient-level data from a clinical trial of its Zilver PTX peripheral paclitaxel-eluting stent.
The move comes a month after the...
Two companies with paclitaxel-coated devices issue corrections to published data
Both Medtronic and Cook Medical have issued corrections to published data regarding the safety of their paclitaxel-coated devices. While Medtronic have revised IN-PACT post-market...
Data presented at LINC confirm the safety and effectiveness of Zilver...
Findings presented at the Leipzig Interventional Course (LINC; 22–25 January 2019, Leipzig, Germany) contradict the results of a meta-analysis published in the Journal of...
IZI Medical acquires Quick-Core biopsy and breast localisation needle assets from Cook...
IZI Medical Products (IZI) has acquired select soft tissue biopsy and breast localisation needle assets from Cook Medical. The acquired portfolio of products is...
New Zilver PTX global data finds 76% freedom from TLR at...
Results from an aggregated data analysis of the use of the Zilver PTX drug-eluting peripheral stent (DES; Cook Medical) in challenging patient populations concluded...
Cook Medical receives FDA approval for first 5mm diameter SFA drug-eluting...
Cook Medical have announced that a new 5mm diameter version of Zilver PTX was approved by the US Food and Drug Administration (FDA). It...
Eluvia outperforms Zilver PTX in IMPERIAL 12-month results
The Eluvia drug-eluting vascular stent system (Boston Scientific) shows superior primary patency compared to the Zilver PTX drug-eluting stent (Cook Medical), concludes the 12-month...
Cook Medical announces successful resolution of 2014 FDA warning letter
Cook Medical has announced that it received a close-out letter from the US Food and Drug Administration (FDA) resolving a 2014 warning letter for...
Cook Medical introduces a new length of Zilver PTX
Cook Medical has introduced the 140mm-length Zilver PTX drug-eluting peripheral stent in both 6 and 7mm diameters in the USA. The longer length comes...
Mark Breedlove and DJ Sirota to lead new Cook Medical business...
Cook Medical has announced the leaders of its two new business divisions. Mark Breedlove has been named vice president of Cook’s Vascular division and...
Cook Medical announces major realignment of its business
Cook Medical has announced key changes that simplify its organisational structure to better support customers.
These changes realign the current sales, marketing, research and...
Vascular plugs score over platinum coils on radiation dose in pelvic...
A prospective, randomised single-centre study that set out to compare the safety and efficacy of vascular plugs and fibred platinum coils for embolization in...
Cook Medical’s inferior vena cava filters fare better than ALN’s in...
Gerard Goh, Radiology, The Alfred Hospital, Melbourne, Australia, reported results from a comparative study of inferior vena cava (IVC) filters at CIRSE 2017 that...
Cook Medical announces Universa sets to streamline percutaneous drainage product line
Cook Medical has rounded out the Universa line with the introduction of two new sets for percutaneous urinary drainage.
Each set includes a catheter and...
Final patient enrolled for VIVO iliofemoral venous stent study
Cook Medical has completed enrolment in the first clinical study of an iliofemoral venous stent conducted in the USA under a US FDA-approved investigational device exemption.
The...
Cook Medical issues global recall of Roadrunner UniGlide hydrophilic wire guides...
The US Food and Drug Administration has announced that Dutch company DSM Biomedical BV—Cook Medical’s supplier of hydrophilic coating for the Roadrunner Uniglide hydrophilic...