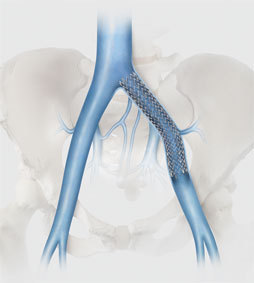
Cook Medical has completed enrolment in the first clinical study of an iliofemoral venous stent conducted in the USA under a US FDA-approved investigational device exemption.
The VIVO clinical study is a prospective, non-randomised, multicentre study intended to evaluate the safety and effectiveness of the Zilver Vena venous stent in the treatment of symptomatic iliofemoral venous outflow obstruction.
A total of 243 patients with acute or chronic symptomatic iliofemoral venous outflow obstruction were enrolled in the VIVO Clinical Study, which has a one-year primary endpoint. The study is being conducted in 29 active sites throughout the US as well as one in Taiwan.
A press release reports that Cook Medical recently completed a similar first-of-its-kind study in the European Union on the safety and performance of the Zilver Vena stent. Gerard O’Sullivan (Galway University Hospitals, Galway, Ireland) presented data from the VIVO-EU study at CIRSE 2016 and VEITHsymposium.
Anthony Comerota (Jobst Vascular Institute, Toledo, USA), one of the study’s principal investigators, comments: “I view the completion of enrolment for this trial as an important milestone in development of options for managing patients with proximal venous obstruction. I am excited to see the full enrollment of the first FDA-approved venous stent trial.”
This has the opportunity to help many patients,” agreed Lawrence “Rusty” Hofmann (Stanford University Hospital, Stanford, USA), co-principal investigator, agreed.













