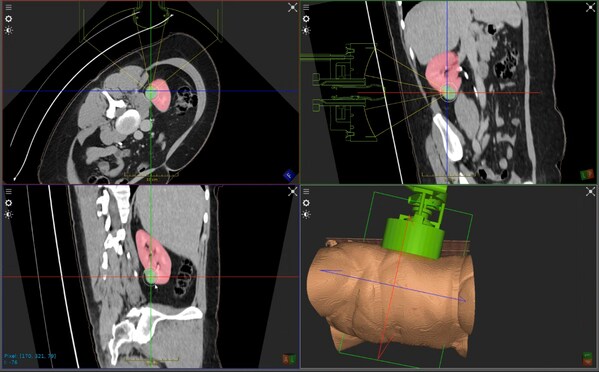
HistoSonics has announced the first kidney patient has been treated using its histotripsy platform. The procedure was performed at the Leeds Teaching Hospitals NHS Trust (Leeds, UK) by Tze Min Wah, senior consultant radiologist and clinical lead for interventional oncology programme. This procedure marks the initial treatment in the HistoSonics-sponsored CAIN trial, which is a Phase I prospective, multicentre study designed to evaluate the safety and technical success of the company’s histotripsy system in targeting and destroying primary solid renal tumours without the need for incisions or needles.
“This first treatment is a significant milestone for the company as it represents expansion into our second active clinical application (after liver) and supports our mission to deliver histotripsy to patients who may potentially benefit from its unique capabilities,” commented Mike Blue, president, and CEO of HistoSonics. Blue added, “our goal is to enable physicians to precisely target and destroy kidney tumours with our novel, non-invasive solution, avoiding the morbidity and complications seen with current invasive surgery or ablative techniques.”
Current kidney therapies such as partial nephrectomy and thermal ablation are invasive and exhibit complications from bleeding and infection that non-invasive histotripsy may avoid. While surgical intervention is the “gold standard” in removing kidney tumours, a non-invasive approach with histotripsy provides the potential to destroy targeted tissue without damaging non-targeted kidney tissue. Additionally, histotripsy’s purely mechanical mechanism of cellular destruction could preserve function of the kidney’s urine-collecting system and eliminate certain complications seen with existing invasive approaches.
Wah commented—”I was delighted to lead the clinical team in carrying out this world’s first kidney tumour treatment using histotripsy and a real privilege to have the trust of the patient and their family in translating this innovative technology into our clinic. The CAIN trial represents a significant milestone for treatment of solid renal tumours with histotripsy as a needle-less technology and is a paradigm shift from this point onwards.”
HistoSonics’ image-guided sonic beam therapy system uses advanced imaging and proprietary sensing technology to deliver non-invasive, personalised treatments with precision and control. The science of histotripsy uses focused sound energy to produce controlled acoustic cavitation that mechanically destroys and liquifies targeted tissue at subcellular levels. The company believes that the novel mechanism of action of their proprietary technology provides significant advantages to patients, including the ability of the treatment site to recover and resorb quickly. Uniquely, the HistoSonics’ platform also provides physicians the ability to monitor the destruction of tissue under continuous real-time visualisation and control, unlike any modality that exists today.
HistoSonics histotripsy systems are not currently available for sale, HistoSonics advises.