Tag: HistoSonics
First patient treated with HistoSonics Edison histotripsy system
HistoSonics, developer of a non-invasive platform and sonic beam therapy called histotripsy, has announced the treatment of the first patient in its pivotal #HOPE4KIDNEY...
HistoSonics receives FDA clearance for Edison histotripsy system
HistoSonics, the manufacturer of the Edison System and novel histotripsy therapy platforms, has announced the marketing authorisation of its “breakthrough” platform via the...
#HOPE4LIVER trial demonstrates safety and efficacy of histotripsy for liver tumours
The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2023 annual meeting (9–13 September, Copenhagen, Denmark) played host to another FIRST@CIRSE session, which saw...
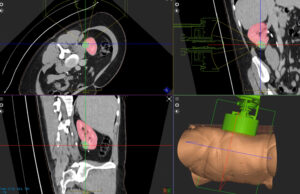
HistoSonics announces first kidney tumour treatment using histotripsy
HistoSonics has announced the first kidney patient has been treated using its histotripsy platform. The procedure was performed at the Leeds Teaching Hospitals NHS...
HistoSonics receives FDA breakthrough device designation for novel sonic beam therapy
HistoSonics has revealed that the US Food and Drug Administration (FDA) has granted the company breakthrough device designation for its new histotripsy targeted liver...