Hansen has announced that it has accelerated the development of the latest addition to the Magellan Robotic System portfolio, the Magellan 3F robotic microcatheter. The development of this important product line expansion has surpassed key milestones and is moving toward the final stages of the development process.
Hansen Medical continues to experience procedural growth with the current Magellan product portfolio and recently surpassed the 1000th procedure performed with the Magellan Robotic System.
“Significant effort has been applied to the development of the Magellan 3Fr Robotic Catheter and it represents a major clinical and commercial opportunity for the organisation,” said Cary Vance, Hansen Medical’s president and chief executive officer. As an appreciably smaller robotic catheter, the Magellan 3Fr may enable physicians to navigate to smaller, more challenging anatomy. Further, this capability may open up larger market opportunities such as applications for neuro and coronary intervention, cancer treatment and limb salvage.
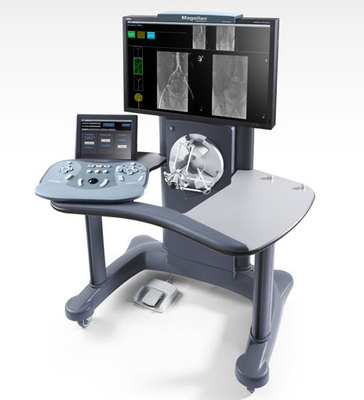
The Magellan Robotic System is drives Magellan Robotic Catheters and guide wires during minimally-invasive, endovascular procedures. Magellan is designed to offer procedural predictability, precision, and catheter stability as physicians navigate inside blood vessels and deliver therapy. Image-guided medical procedures using interventional fluoroscopy, while growing rapidly, are the leading source of occupational ionizing radiation exposure for medical personnel. Magellan’s remote workstation allows physicians to control robotic catheters and guide wires while seated away from the radiation field, which has been shown to reduce radiation exposure for the physician by as much as 95% in complex endovascular procedures.













