Jamie Bell
Positive NOVARAD study results could herald “new era” for radiation protection
Cathpax—a spinoff of the Lemer Pax group that designs, develops and commercialises team-wide, full-body radiation protection systems for interventional medicine practitioners—has today announced positive...
Polyembo announces close of funding to boost new vascular embolisation technology
Polyembo—a medical device company founded to develop the Scrunchy, Sphere, PuffyCoil and other vascular embolisation technologies—has announced the closing of a new round of...
Boston Scientific recalls Carotid Wallstent Monorail devices over “manufacturing defect”
Boston Scientific has recalled its Carotid Wallstent Monorail endoprosthesis owing to a “manufacturing defect” that has led to devices having an inner lumen that...
Terumo Neuro receives US FDA approval for carotid stent system
Terumo Neuro has announced that its carotid stent system has received premarket approval (PMA) from the US Food and Drug Administration (FDA).
This milestone marks...
Medtronic expands US carotid market presence with exclusive Contego distribution agreement
Medtronic announced today that it has entered into an exclusive US distribution agreement with Contego Medical. Under the agreement, Medtronic will be the sole...
Arsenal Medical launches EMBO-02 study to assess NeoCast in cSDH treatment
Arsenal Medical today announced the initiation of the EMBO-02 clinical study of NeoCast in the treatment of chronic subdural haematomas (cSDHs). NeoCast is a...
Penumbra announces completion of enrolment in THUNDER IDE study
Penumbra has announced the completion of enrolment in its THUNDER investigational device exemption (IDE) clinical study for patients with acute ischaemic stroke.
THUNDER is evaluating...
Microvention officially rebrands to Terumo Neuro, reflecting “expanded focus and strategic...
Microvention, a wholly owned subsidiary of Terumo Corporation, has today announced its official rebranding to Terumo Neuro, effective immediately.
This name change signifies a new...
Gravity launches Supernova stent retriever and Neutron aspiration catheter for global...
Gravity Medical Technology has announced the launch of its next-generation stroke treatment devices: the Neutron aspiration catheter and the Supernova stent retriever. Early adoption...
Novel liquid embolic agent meets primary feasibility and safety endpoints in...
Arsenal Medical has announced that NeoCast—a first-of-its-kind, shear-responsive liquid embolic material designed for deep distal penetration—met its primary feasibility and safety endpoints in the...
Imperative announces clinical findings from pivotal trial evaluating Zoom reperfusion system
Imperative Care has announced that late-breaking data from the Imperative trial were presented recently at the ongoing Society of NeuroInterventional Surgery (SNIS) annual meeting...
Imperative announces US FDA clearance and initial cases with “first” stroke-specific...
Imperative Care has announced US Food and Drug Administration (FDA) 510(k) clearance of its Zoom 6Fr insert catheters, the company’s latest innovation in elevating...
Penumbra launches Midway intermediate catheters and expands European stroke portfolio
Today, Penumbra announced the launch of its Midway 43 and Midway 62 delivery catheters, which are designed to provide ideal tracking and a stable...
Questions linger: BASIL-3 does not find for drug-eluting technologies for CLTI
In the UK National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA)-funded BASIL-3 randomised controlled trial (RCT), neither drug-coated balloon (DCB)...
“We know which DCB to use”—but many other questions remain in...
A first-time presentation of five-year data from the IN.PACT AV Access study evaluating the IN.PACT AV (Medtronic) drug-coated balloon (DCB) today led principal investigator...
Silk Road adds Enroute NPS Plus device to TCAR portfolio
Silk Road Medical has announced the launch of its Enroute transcarotid neuroprotection system Plus (NPS Plus), which the company describes as a key component...
Early DAPT, statin therapy and stent patency among predictors of good...
An analysis involving some 300 patients with anterior-circulation acute ischaemic strokes caused by tandem lesions has determined a number of factors that may be...
Single-centre analysis suggests improved longer-term outcomes with endarterectomy versus TCAR
A large, single-centre analysis has found that, despite parity between the two procedures in terms of perioperative clinical outcomes, carotid endarterectomy (CEA) was associated...
Nanoflex Robotics installs its first remote-ready robotics system in North America
Switzerland-based startup firm Nanoflex Robotics has announced that it recently installed its first remote-ready robotics system for neurovascular procedures at the Jacobs Institute (Buffalo,...
Silk Road expands TCAR portfolio with launch of tapered Enroute system
Silk Road Medical has announced the launch of its tapered Enroute transcarotid stent system to hospitals in the USA, expanding upon the company’s prior...
Sensome initiates trial assessing tissue microsensor technology in peripheral artery disease
Sensome has announced enrolment of the first patients into a feasibility clinical study using the Clotild smart guidewire in peripheral artery disease (PAD). Clotild...
Mentice gains US FDA 510(k) clearance for Ankyras software
Mentice recently announced that Ankyras, the company’s clinical decision support application, has received 510(k) clearance from the US Food and Drug Administration (FDA). The...
Rescue stenting shows good outcomes among Middle Eastern, African and Asian...
Rescue stent placement following a failed thrombectomy procedure has demonstrated “good outcomes” and a low risk of clinically significant bleeding in a multiethnic cohort...
InspireMD presents positive 30-day follow-up results from C-GUARDIANS clinical trial
InspireMD recently presented 30-day results from the C-GUARDIANS US investigational device exemption (IDE) clinical trial evaluating its CGuard embolic prevention stent (EPS) system for...
Contego announces low one-year stroke rates in PERFORMANCE II carotid stent...
Contego Medical has announced the presentation of late-breaking clinical results from the PERFORMANCE II carotid stent trial at the annual Vascular Interventional Advances (VIVA)...
Surmodics launches Preside hydrophilic coatings for neurovascular, coronary and peripheral applications
Surmodics has announced the commercial launch of its most advanced hydrophilic medical device coating technology, the Preside hydrophilic coatings.
As per a company press release,...
Venous Summit 2023: Holistic, multidisciplinary approach to venous care is “mandatory”
This advertorial, sponsored by BD, is intended only for readers outside the USA.
“A dedicated, holistic venous approach is mandatory—including deep, superficial and pelvic venous...
Robocath completes CARE clinical trial evaluating robotic carotid stenting
Robocath has today announced the results of its CARE clinical trial focusing on robotic carotid stenting—the first phase of a research programme launched in...
Rates of stroke, death and myocardial infarction post-TCAR do not differ...
While sex disparities regarding the outcomes of carotid revascularisation have “long been a concern”, new prospective data published in the Journal of Vascular Surgery...
Artivion announces meaningful all-cause mortality reduction in AMDS PERSEVERE trial
Artivion has announced presentation of interim results from the Ascyrus medical dissection stent (AMDS) PERSEVERE clinical trial in a late-breaking science session at the...
“No significant difference” between aspiration and stent-retriever thrombectomy in medium vessel...
When utilised as a first-line technique for medium vessel occlusion (MeVO) stroke, aspiration and stent-retriever thrombectomy have demonstrated “no significant difference” in imaging-related or...
Microvention announces commercial availability of Eric retrieval device in the USA
Microvention, a wholly owned subsidiary of Terumo, has announced that the Eric retrieval device is now commercially available in the USA for ischaemic stroke...
Vision recovery among potential benefits of endovascular therapy in PCA occlusion...
A multicentre study presented at the recent European Stroke Organisation Conference (ESOC; 24–26 May, Munich, Germany) has found that vision recovery and an increased...
Penumbra expands computer-aided thrombectomy offering with Lightning Bolt 7 launch
Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of Lightning Bolt 7, which the company claims is the most...
Penumbra launches new RED 43 reperfusion catheter for mechanical thrombectomy
Penumbra recently announced the launch of the latest addition to its RED family of catheter devices for mechanical thrombectomy, the RED 43 reperfusion catheter.
According...
Adept launches Adducted Arm Scoop product for use during image-guided procedures
Adept Medical has launched the Adducted Arm Scoop—an improved solution for supporting the adducted arms of supine patients during image-guided procedures.
Adept’s new, “extremely durable”...
UK MHRA awarded £10 million to fast-track patient access to medical...
A total of £10 million has been awarded to the Medicines and Healthcare products Regulatory Agency (MHRA)—an executive agency of the UK Department of...
Study finds “dismally low” global access to thrombectomy and “enormous disparity”...
The Society of Vascular and Interventional Neurology (SVIN) has released its first global analysis of access to mechanical thrombectomy for the treatment of large...
Distal embolic protection linked to significantly better outcomes in carotid stenting
Distal embolic protection using a filter has been associated with improved transfemoral carotid artery stenting (tfCAS) outcomes in terms of in-hospital stroke and death...
Thrombectomy appears comparable to medical management in treating ACA occlusion stroke
Mechanical thrombectomy appears to be a safe and technically feasible treatment option for ischaemic strokes caused by primary isolated anterior cerebral artery (ACA) occlusions...
Synchron announces publication of brain-computer interface clinical trial in JAMA Neurology
Synchron has announced that the medical journal JAMA Neurology has published peer-reviewed, long-term safety results from a clinical study in four patients with severe...
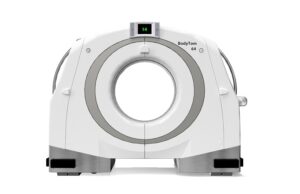
Neurologica announces US FDA 510(k) clearance of BodyTom 64
Neurologica, a subsidiary of Samsung Electronics, has announced that its head-to-toe trauma imaging solution—the BodyTom 64 point-of-care mobile computed tomography (CT) scanner—has received 510(k) clearance...
Swiss parliament votes to accept US FDA-approved medical devices
The Swiss Federal Assembly has voted in favour of accepting medical devices with US Food and Drug Administration (FDA) marketing authorisation in Switzerland.
A motion...
Xeltis starts pivotal clinical trial of “first-ever” restorative synthetic haemodialysis access...
Xeltis announced today the initiation of a pivotal trial with the “first-ever” restorative synthetic haemodialysis access grafts, dubbed ‘Axess’.
The first two patients have been...
Effective alternatives to surgery for renal cell carcinomas discussed at CIRSE...
The management of renal cancer patients featured prominently at this year’s Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual meeting (10–14 September, Barcelona,...
Synchron to begin COMMAND trial enrolment at University of Pittsburgh
Synchron has announced that enrolment in the COMMAND trial has commenced at the University of Pittsburgh in Pittsburgh, USA.
The COMMAND trial is an early...
Transit Scientific announces US FDA clearance for XO RX angioplasty platform
Transit Scientific has announced that the XO RX 2.2Fr and XO RX 3.8Fr platform has received US Food and Drug Administration (FDA) clearance to...
Xeltis presents “promising” first-in-human data on restorative haemodialysis access graft
Xeltis today announced what it describes as “very promising” preliminary efficacy and safety results from one of the centres participating in the AXESS study—a...
Penumbra announces European launch of RED reperfusion catheters for stroke care
Penumbra announced today that its RED reperfusion catheters have secured a CE mark and are now commercially available in Europe. The catheters are part of...
Phase 2 trial demonstrates potential of medication-free treatment for metastatic kidney...
Building upon “pioneering work” at University of Texas Southwestern (UTSW) Medical Center (Dallas, USA), investigators recently reported the results of a clinical trial exploring the role of...
AVeVA study confirms benefit of covered stent placement in graft-vein anastomotic...
A prospective, multicentre study involving the Covera vascular covered stent (BD) has confirmed the benefits of immediate, post-percutaneous transluminal angioplasty (PTA) placement of the...
Bluegrass Vascular announces Journal of Vascular Surgery paper reporting use of...
Bluegrass Vascular Technologies recently announced the publication of a paper featured on the cover of the June 2022 issue of the Journal of Vascular Surgery (JVS). The paper...
Artio Medical closes US$28 million in additional Series A financing
Artio Medical today announced the closing of US$28 million in oversubscribed Series A2 and Series A3 financing, bringing the total amount raised by the company to...
Adept announces Prone Support product launch
Adept Medical says it has responded to the call from interventional radiologists for a device to comfortably support and manage a patient in the...
Medtronic names Laura Mauri as new chief scientific, medical and regulatory...
Medtronic has announced that Laura Mauri has been appointed as the company’s chief scientific, medical and regulatory officer. This appointment adds to Mauri's prior responsibilities...
Corindus relocates Massachusetts headquarters to accommodate company growth
Corindus, a Siemens Healthineers company, announced today the opening of its new headquarters at 275 Grove Street in Newton, USA. Siemens Healthineers relocated the...
Synchron announces long-term safety results with Stentrode device for severe paralysis
Synchron has announced the results from a study in which four people with amyotrophic lateral sclerosis (ALS) received an implant of the company’s Stentrode...
Nipro to bring Cronus high-pressure PTA balloon catheter to USA
Nipro Medical Corporation is set to introduce its second-generation Cronus high-pressure (HP) percutaneous transluminal angioplasty (PTA) balloon catheter to the US market.
Cronus HP is...
Implantable brain-computer interface enables completely locked-in patient to communicate
Researchers at the Wyss Center for Bio and Neuroengineering (Geneva, Switzerland), in collaboration with the University of Tübingen (Tübingen, Germany), have enabled a person...
A closer look at new technologies in malignant ureteric obstruction
Malignant ureteric obstruction (MUO) poses several tangible threats to cancer patients—and these threats translate, in turn, to challenges for the interventional radiologist and other...
Fist Assist Devices announces Australian distribution deal and sales launch
Fist Assist Devices has announced a three-year affiliation with Regional Health Care Group to commercialise and launch sales of the Fist Assist Model FA-1...
Largest real-world experience to date with VasQ device indicates long-term benefits...
The VasQ external support device (Laminate Medical) has demonstrated long-term benefits for radiocephalic (forearm) arteriovenous fistula (AVF) creation in a retrospective analysis of 150...
Perfuze raises €22.5m in Series A funding for novel acute ischaemic...
Perfuze announced today that it has closed a €22.5 million Series A investment round—the proceeds from which will be used to drive the next...
Shifting paradigms in diagnosing and treating small renal masses
In light of recent and upcoming developments in the space, Vinson Wai-Shun Chan and Tze Min Wah (Leeds, UK) explore the evidence and latest...
Mechanical thrombectomy shown to restore more function than medication alone following...
A new study from Japan has become the first randomised controlled trial (RCT) to demonstrate the effectiveness of endovascular mechanical thrombectomy procedures in patients...
Cerenovus launches Emboguard balloon guide catheter for acute ischaemic stroke
Cerenovus—a neurovascular firm that forms part of Johnson & Johnson Medical Devices Companies—has announced the launch of Emboguard, its next-generation balloon guide catheter to...
NovaGuide intelligent ultrasound found to significantly improve right-to-left shunt detection
NovaSignal Corporation has announced the results of a multicentre, prospective, single-arm study indicating that the company’s autonomous NovaGuide intelligent ultrasound is three times more...
Imperative launches Zoom POD aspiration tubing for use in stroke treatments
Imperative Care has announced the launch of its Zoom POD aspiration tubing—the company’s latest innovation in elevating stroke care.
This is the newest addition...
ISC 2022: Global EXCELLENT study “shows how much stroke thrombectomy has...
Mechanical removal of blood clots reduced post-stroke disability in nearly half of “all-comer” real-world stroke patients in a global study, according to preliminary late-breaking...
FDA issues two final guidances for including patient perspectives in medical...
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As...
IMPRESSION trial assessing MagicTouch AVF passes 50% enrolment
Concept Medical has shared the latest update from its IMPRESSION (Sirolimus-coated balloon angioplasty versus plain balloon angioplasty in the treatment of dialysis access dysfunction)...
Fist Assist receives FDA Breakthrough Device designation for wearable vein dilation...
Fist Assist Devices has announced that it recently received Breakthrough Device designation from the US Food and Drug Administration (FDA) for its Fist Assist...
Arrow recalls percutaneous thrombolytic device kits over risk of separation
Arrow International has recalled its Arrow-Trerotola over-the-wire 7Fr percutaneous thrombolytic device (PTD) kits, which are used to remove clots in adult patients who have...
Amplifi vein dilation system demonstrates “encouraging” clinical results in haemodialysis access
Artio Medical has announced that full results from its first-in-human clinical study of the Amplifi vein dilation system were presented at the 2021 VEITHSymposium...
The Holmium Platform: Three integrated products delivering individualised SIRT at its...
This educational supplement, sponsored by Terumo Interventional Systems, is intended for readers in Europe only.
Highlights:
Ana Oliveira (Centro Hospitalar Universitário de São João, Porto,...
Rapid Medical to initiate trial expanding thrombectomy treatment across distal regions...
Rapid Medical today announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for what it claims is the first-ever trial to...
Benefits of percutaneous fistula creation further bolstered by five-year Ellipsys data
The Ellipsys vascular access system (Avenu Medical/Medtronic) can be used to easily and safely create durable percutaneous arteriovenous fistulas (pAVFs) for haemodialysis—with new, long-term...
Medtronic unveils data on hypertension treatment preferences, launches SPYRAL AFFIRM study
Medtronic today announced findings from a new study of patient preferences for the treatment of hypertension. The findings are set to be presented during...
“Very encouraging” 12-month data from WRAPSODY FIRST study presented at CIRSE...
Twelve-month results from a first-in-human study of the Wrapsody cell-impermeable endoprosthesis (Merit Medical Systems) for the treatment of access circuit stenosis in haemodialysis patients...
Results from first-in-human study of Amplifi vein dilation system presented at...
Preliminary clinical results from a first-in-human study assessing the Amplifi vein dilation system (Artio Medical) were presented at Vascular Interventional Advances (VIVA) 2021 (5–7 October...
Medtronic receives CE mark approval for radial artery access portfolio
Medtronic today announced it has received CE mark approval for its radial artery access portfolio, which includes the Rist radial access selective catheter and the Rist...
Phenox highlights benefits of longer stentriever devices with new study results
Following its acceptance into Frontiers in Neurology, Phenox has announced the results of a paper exploring the benefit of choosing a longer stentriever for...
SNIS 2021: Remote stroke interventions raise several unique ethical challenges
At the Society of NeuroInterventional Surgery’s 18th annual meeting (SNIS; July 26–29 2021, Colorado Springs, USA and virtual), Jeffrey Saver, professor of Neurology at...
Artio Medical completes enrolment of first-in-human trial investigating Amplifi vein dilation...
Artio Medical announced today it has completed enrolment in its first-in-human study evaluating the Amplifi vein dilation system. In the study, five patients were treated...
Study shows increased risk of renal failure following lytic therapy for...
A large, single-centre retrospective study has revealed the risk of acute kidney injury (AKI) following pharmacomechanical thrombolysis (PMT) for lower extremity deep vein thrombosis...
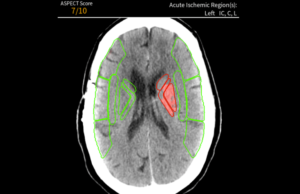
Avicenna.AI receives CE Mark for AI tool to assess stroke severity
Medical imaging AI specialist Avicenna.AI has received CE mark certification for its CINA ASPECTS AI tool for stroke severity assessment. CINA ASPECTS automatically processes...
More mixed results for DCBs in AV access maintenance as evidence...
Additional randomised controlled data regarding the effectiveness of drug-coated balloons (DCBs) in maintaining arteriovenous (AV) access offers new evidence, but it is freighted with...
Bifurcated T-stent reconstruction is safe and effective treatment for HVOO in...
A study has found that bifurcated T-stent reconstruction offers a safe and effective treatment option for hepatic venous outflow obstruction (HVOO) with anastomotic stenoses...
Results of two-year study show Zilver Vena Venous Stent is safe...
The results of a two-year study support the continued safety and effectiveness of the Zilver Vena Venous Stent (Cook Medical) in treating symptomatic iliofemoral...
Fluoroscopically-guided insertion of td-CVC found to be “low exposure”, despite large...
While there is large variability based on the site of insertion, patient characteristics and previous accesses, fluoroscopically-guided insertion of tunnelled central venous catheters (td-CVC)...