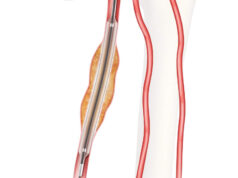
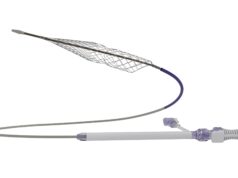
Vesalio has announced the initiation of a prospective, single-arm, multicentre study supporting the recently launched pVasc thrombectomy system for non-surgically removing peripheral occlusions. This multidisciplinary clinical research initiative aims to collect real-world insights on the pVasc system in treating patients suffering from peripheral arterial disease (PAD) that may lead to acute limb ischaemia (ALI).
A press release notes that current thrombectomy tools encounter multiple challenges, including difficulty accessing smaller arteries, prolonged time to flow restoration, and the risk of distal embolisation. “The pVasc system, featuring next-generation Drop Zone technology and a unique, low-profile design, is specifically engineered to address these limitations,” the company states. “The pVasc study will generate vital real-world evidence to support the optimisation of revascularisation techniques and outcomes for patients with PAD-related conditions such as ALI.
Vesalio also shares that the company will be attending the TCT Conference (27–30 October, Washington DC, USA), VIVA (3–6 November, Las Vegas, USA), and VEITHsymposium (19–23 November, New York, USA) in the coming weeks.
“The recent launch of pVasc in the USA represents a major milestone in our commitment to innovating to improve patient outcomes in vascular occlusion,” said Steve Rybka, chief executive officer of Vesalio. “We expect the post-market collection of real-world data to further validate the capabilities of our technology in addressing the challenges of peripheral vascular disease. We continue to support physicians with both technology and data to achieve the best possible outcomes for their patients.”