Tag: thrombectomy
Boston Scientific announces agreement to acquire Penumbra
Boston Scientific and Penumbra today announced the companies have entered into a definitive agreement under which Boston Scientific will acquire Penumbra in a cash...
Medtronic announces first commercial use of Liberant thrombectomy system
Medtronic has announced the first commercial use of its Liberant thrombectomy system, which is indicated for the removal of fresh, soft emboli or thrombi...
Penumbra receives CE mark for Lightning Bolt 12 and Lightning Bolt...
Penumbra recently announced that it has secured CE-mark approval for the Lightning Bolt 12 and Lightning Bolt 6X with TraX, which the company notes...
Penumbra receives CE mark for Lightning Bolt 12 and Lightning Bolt...
Penumbra recently announced that it has secured CE-mark approval for the Lightning Bolt 12 and Lightning Bolt 6X with TraX, which the company notes...
Vesalio receives two US FDA 510(k) clearances for aspiration devices
Vesalio recently announced two new US Food and Drug Administration (FDA) 510(k) clearances for its aspiration devices, designed for peripheral and neurovascular applications.
By integrating...
New Medicare analysis shows increased thrombectomy use in venous disease
A new review of Medicare data shows a marked increase for both arterial and venous thrombectomy claims for venous thromboembolic disease filed between 2017–2022.
Researchers...
BD enrols first patient in XTRACT registry of Rotarex catheter system...
BD has announced the enrolment of the first patient in the XTRACT registry, a prospective, multicentre, single-arm, postmarket registry study designed to evaluate the...
SYMPHONY-PE trial demonstrates positive efficacy, efficiency, and safety results
Imperative Care has announced efficacy and safety results from the pivotal SYMPHONY-PE trial evaluating the company’s Symphony thrombectomy system in the treatment of acute...
Imperative Care receives FDA 150(k) clearance for Symphony thrombectomy system
Imperative Care has today announced US Food and Drug Administration (FDA) 510(k) clearance of its Symphony thrombectomy system to treat pulmonary embolism (PE).
This clearance...
Inari Medical, now part of Stryker, launches InThrill thrombectomy system
Inari Medical—now part of Stryker—has today announced the launch of its next-generation InThrill thrombectomy system.
The company has stated in a recent press release that...
First patient treated with InterVene’s Recana thrombectomy catheter system
InterVene announced today that the first patient has been treated with its Recana thrombectomy catheter system for venous in-stent restenosis (ISR).
The company shares...
Endovascular Engineering’s ENGULF pivotal trial completes patient enrolment
Endovascular Engineering (E2) has completed patient enrolment in the pivotal cohort of its ENGULF trial, involving the Hēlo pulmonary embolism (PE) thrombectomy system.
The investigational...
Penumbra’s STORM-PE randomised trial completes enrolment
Penumbra has announced the completion of enrolment in the STORM-PE clinical trial.
The pivotal, prospective, multicentre randomised controlled trial enrolled 100 patients to evaluate computer...
New THRIVE data on Penumbra’s CAVT technology for acute limb ischaemia...
Recent THRIVE study data show that Penumbra’s computer-assisted vacuum thrombectomy (CAVT) technology not only has the potential to improve outcomes for lower extremity acute...
Imperative Care announces completion of enrollment in the SYMPHONY-PE study
Imperative Care today announced the completion of patient enrolment in its SYMPHONY-PE study, a pivotal investigational device exemption (IDE) trial evaluating the safety and...
JETi registry reports effective thrombus reduction in lower extremity DVT
Recently presented at the Society of Interventional Radiology (SIR) annual scientific meeting (29 March–2 April, Nashville, USA), data from the Jet Enhanced Thrombectomy intervention...
Computer-assisted vacuum thrombectomy device demonstrates “highly” improved device use time
In a subgroup analysis of the STRIKE-PE study regarding patients treated with the latest iteration of Penumbra’s 16Fr Lighting Flash computer-assisted vacuum thrombectomy (CAVT)...
Surmodics announces commercial release of Pounce XL thrombectomy system
Surmodics has announced the commercial release of the Pounce XL thrombectomy system, the latest in a suite of Pounce thrombectomy systems that provide endovascular...
VentiV Scientific mechanical thrombectomy system granted FDA approval
VentiV Scientific has announced the US Food and Drug Administration (FDA) clearance of VentiV mechanical thrombectomy system to remove blood clots from the peripheral...
Imperative Care expands Symphony thrombectomy portfolio with new US FDA approval
Imperative Care today announced US Food and Drug Administration (FDA) 510(k) clearance of the 82cm version of its Symphony 16Fr catheter, the company’s latest...
Inari Medical, now part of Stryker, launches Artix thrombectomy system
Inari Medical, now part of Stryker, recently announced the launch of its Artix thrombectomy system. Purpose-built for the distinct needs of the peripheral arterial...
Akura Medical enrols first patient in QUADRA-PE study
Akura Medical has announced today the first patient enrolment in the QUADRA-PE study evaluating the Katana thrombectomy system in patients with acute pulmonary embolism...
Surmodics announces successful early clinical use of Pounce XL thrombectomy system
Surmodics has announced the successful early clinical use of its Pounce XL thrombectomy system. The device received US Food and Drug Administration (FDA) 510(k)...
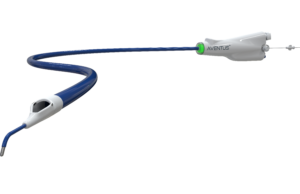
AVENTUS trial of thrombectomy system for PE treatment completes enrolment
Inquis Medical has announced completion of patient enrolment in its AVENTUS clinical trial, a pivotal investigational device exemption (IDE) trial evaluating the safety and...
Argon Medical enrols first patient in CLEAN-PE study
Argon Medical announces the first patient enrolment in the CLEAN-PE study. The prospective, multicentre CLEAN-PE study aims to evaluate the safety and efficacy of...
Surmodics announces early results from PROWL registry of Pounce thrombectomy system
Surmodics has announced that early results of a subset of 60 real-world acute, subacute, and chronic limb ischaemia patients from its PROWL registry study...
PEERLESS trial finds large-bore mechanical thrombectomy superior for intermediate-risk PE
Findings from the first international randomised controlled trial (RCT) to compare patient outcomes following treatment with large-bore mechanical thrombectomy (LBMT) versus catheter-directed thrombolysis (CDT)...
Vesalio announces clinical study initiative for recently launched pVasc thrombectomy system
Vesalio has announced the initiation of a prospective, single-arm, multicentre study supporting the recently launched pVasc thrombectomy system for non-surgically removing peripheral occlusions. This...
Surmodics receives FDA 510(k) clearance for Pounce XL thrombectomy system
Surmodics has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
The Pounce XL thrombectomy...
Argon Medical announces launch of peripheral venous thrombectomy system
Argon Medical has today announced the launch of the CLEANER Vac thrombectomy system for the removal of blood clot from the peripheral venous vasculature.
The...
Penumbra receives CE mark for Lightning Flash 2.0 and Lightning Bolt...
Penumbra today announced it has secured CE mark in Europe for two computer-assisted vacuum thrombectomy (CAVT) technologies—Lightning Flash 2.0 and Lightning Bolt 7.
“Based on...
FlowPhysix announces partnership with 3comma for global distribution of thrombectomy device
FlowPhysix, formerly known as Expanse ICE, has announced a strategic partnership with 3comma Medical who will serve as their international commercial partner for their...
Vesalio launches thrombectomy system in the USA for peripheral occlusions
Vesalio announces the US commercialisation of pVasc thrombectomy system for non-surgically removing peripheral occlusions.
A recent press release outlined that pVasc targets the full...
Thrombectomy gold rush set to follow ATTRACT study, experts argue
At the recent Endo Vascular Access (EVA) meeting (14–15 June, Patras, Greece), Ziv Haskal (University of Virginia School of Medicine, Charlottesville, USA) spoke to...
Novel thrombectomy system demonstrates positive safety and feasibility results in treating...
Late-breaking data from the ENGULF trial showed that a novel dual-action thrombectomy device was effective and safe in treating acute pulmonary embolism (PE). The...
Expanse Ice aspiration system receives US FDA clearance for vessels of...
Expanse Ice recently announced that its Ice aspiration system has received 510(k) clearance from the US Food and Drug Administration (FDA).
A press release notes...
New data show Penumbra’s Indigo aspiration system used in a single...
During the Society of Interventional Radiology (SIR) 2024 annual meeting (23–28 March, Salt Lake City, USA), newly presented data from a subgroup analysis of...
Early DAPT, statin therapy and stent patency among predictors of good...
An analysis involving some 300 patients with anterior-circulation acute ischaemic strokes caused by tandem lesions has determined a number of factors that may be...
Innova Vascular announces early commercial use of Laguna thrombectomy system
Innova Vascular has announced successful early commercial use of the company's Laguna thrombectomy system. Physicians at University of California (UCLA) Medical Center in Los...
Surmodics announces successful early clinical use of Pounce LP thrombectomy system
Surmodics has announced successful early clinical use of the company’s Pounce LP (low-profile) thrombectomy system. The Pounce LP system, which received US Food and Drug...
Surmodics announces successful early clinical use of Pounce LP thrombectomy system
Surmodics has announced successful early clinical use of the company’s Pounce LP (low-profile) thrombectomy system. The Pounce LP system, which received US Food and Drug...
Inari Medical announces first patient enrolment in PEERLESS II randomised controlled...
Inari Medical has announced the first patient enrolment in PEERLESS II. This prospective, global, multicentre randomised controlled trial (RCT) compares the outcomes of intermediate-risk...
First patient enrolled in STORM-PE RCT evaluating Penumbra’s Lightning Flash for...
Penumbra today announced that the first patient has been enrolled in STORM-PE, a prospective, multicentre, randomised controlled trial (RCT) evaluating anticoagulation alone versus anticoagulation...
“Rapid improvement” in right ventricular metrics following computer-aided mechanical aspiration thrombectomy
Presenting real-world population data in patients with pulmonary embolism (PE), John Moriarty (University of California, Los Angeles Medical Center, Los Angeles, USA) demonstrated results...
Interventional News’ top 10 most popular stories of August 2023
Interventional News’ most popular stories from August include a commentary on intra-arterial approaches to advanced pancreatic cancer; perspectives on 25 years of day-case IR...
Surmodics receives 510(k) clearance for Pounce LP thrombectomy system
Surmodics announced in a press release that it has received US Food and Drug Administration (FDA) 510(k) clearance for its Pounce LP (low...
Inari Medical announces commercial launch of RevCore and Triever16 Curve for...
Inari Medical today announced the launch of two new purpose-built products, the RevCore thrombectomy catheter, and the Triever16 Curve catheter.
According to a company press...
Mechanical thrombectomy: ClotTriever offers “extended window” for DVT treatment
This advertorial, sponsored by Inari Medical, is only available in selected countries and geographies.
During a recent webinar hosted by Inari Medical, a multidisciplinary group...
First patient enrolled in PROWL registry study using Pounce thrombectomy system
Surmodics has announced enrolment of the first patient in PROWL, the Pounce thrombectomy system retrospective registry. PROWL is an open-label, retrospective, multicentre US registry...
Study finds “dismally low” global access to thrombectomy and “enormous disparity”...
The Society of Vascular and Interventional Neurology (SVIN) has released its first global analysis of access to mechanical thrombectomy for the treatment of large...
RESCUE trial shows reduction in segmental and main pulmonary artery occlusions
Robert A Lookstein, who is executive vice chair, Diagnostic, Molecular and Interventional Radiology at the Icahn School of Medicine at Mount Sinai Hospital (New...
Cardiovascular Systems announces US FDA 510(k) submission of Innova Vascular’s thrombectomy...
Cardiovascular Systems (CSI) announced that Innova Vascular (Innova) has submitted a 510(k) premarket notification to the US Food and Drug Administration (FDA) for its...
New mechanical thrombectomy systems unlock possibilities for the treatment of venous...
This advertorial, sponsored by Inari Medical, is only available in selected countries and geographies.
Mechanical thrombectomy with the ClotTriever and FlowTriever systems (Inari Medical) opens...
Penumbra and Asahi Intecc partner to introduce Indigo System to Japan
Penumbra and Asahi Intecc, a Japanese medical device manufacturer, announced that they will collaborate to introduce Penumbra’s Indigo Aspiration System into the Japanese market...
AngioDynamics announces FDA clearance of expanded indications for Auryon atherectomy system
AngioDynamics recently announced that it has received US Food and Drug Administration (FDA) 510(k) clearance of an expanded indication for the Auryon atherectomy system...
Pounce thrombectomy system first-in-human data show 100% technical success in early...
Surmodics has announced that its Pounce thrombectomy system achieved 100% technical success in 20 first-in-human (FIH) procedures. The FIH data were presented by Gary...
Profligacy and dangerous misconceptions in dialysis access
Scott Trerotola (University of Pennsylvania, Philadelphia, USA), who will be awarded a Society of Interventional Radiology (SIR) Gold Medal in June 2022, raises some...
Penumbra announces the European launch of the Indigo system with Lightning...
Penumbra has announced that its Indigo aspiration system with Lightning 7 and Lightning 12 have secured CE mark and are now commercially available in...
Truvic announces US FDA 510(k) clearance for Prodigy thrombectomy system
Truvic Medical, a subsidiary of Imperative Care, announced in a press release that it has received 510(k) clearance from the US Food and Drug...
Cordis makes strategic investment in E2, a developer of next-generation thrombectomy...
Cordis has announced a strategic investment venture that will expand the scope of the global cardiovascular technology company into the venous thromboembolism (VTE) market...
Cardiovascular Systems partners with Innova Vascular to develop full line of...
Cardiovascular Systems Inc (CSI) recently announced it has partnered with Innova Vascular (Innova) to develop a full line of novel thrombectomy devices.
According to a...
AVF 2022: Six-month CLOUT data indicate ClotTriever can effectively remove full...
Six-month outcomes from the ongoing CLOUT registry demonstrate the “safety and efficacy” of the ClotTriever thrombectomy system (Inari Medical) in a real-world deep vein...
Mechanical thrombectomy shown to restore more function than medication alone following...
A new study from Japan has become the first randomised controlled trial (RCT) to demonstrate the effectiveness of endovascular mechanical thrombectomy procedures in patients...
ISC 2022: Global EXCELLENT study “shows how much stroke thrombectomy has...
Mechanical removal of blood clots reduced post-stroke disability in nearly half of “all-comer” real-world stroke patients in a global study, according to preliminary late-breaking...
Akura Medical closes US$25M Series A1 funding round
Akura Medical has announced the closing of its $25M Series A1 financing, which will be used to support the development of its next-generation thrombectomy device.
The financing...
Rapid Medical to initiate trial expanding thrombectomy treatment across distal regions...
Rapid Medical today announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for what it claims is the first-ever trial to...
Phenox highlights benefits of longer stentriever devices with new study results
Following its acceptance into Frontiers in Neurology, Phenox has announced the results of a paper exploring the benefit of choosing a longer stentriever for...
Surmodics announces successful first patient use of Pounce thrombectomy system
Surmodics has announced that J. Michael Bacharach, a vascular interventionalist/cardiologist at North Central Heart, a division of Avera Heart Hospital in Sioux Falls, USA,...
AngioDynamics receives 510(k) clearance for AlphaVac mechanical thrombectomy system
AngioDynamics recently announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the AlphaVac mechanical thrombectomy system.
According to...
Mermaid Medical Group reports full commercialisation of the D-Clot HD rotational...
Mermaid Medical Group recently announced US commercial availability of the D-Clot HD rotational thrombectomy system. As of 12 January, the company reports 25 successful...
Philips launches QuickClear mechanical thrombectomy system for blood clot removal in...
Royal Philips has announced the launch of the QuickClear mechanical thrombectomy system. The single-use system delivers an all-in-one aspiration pump and catheter to remove...
Veryan Medical to support commercialisation of Jeti thrombectomy system in Germany
It was recently announced that Veryan Medical will support Walk Vascular in the commercialisation of the Jeti peripheral thrombectomy system in Germany.
Walk Vascular’s Jeti...
FDA clears Aspire MAX mechanical thrombectomy system
Control Medical Technology has announced that the US Food and Drug Administration (FDA) has cleared its Aspire MAX 7 – 11F mechanical thrombectomy...
Training IRs to perform thrombectomies maintains quality of stroke care and...
Training interventional radiologists to perform endovascular thrombectomies results in positive outcomes for patients experiencing stroke, according to a study presented at the Society of...
Preliminary in vitro data is promising for Anaconda Biomed’s advanced thrombectomy...
Preliminary in vitro data on Anaconda Biomed’s advanced thrombectomy system showed that the reperfusion rates with this novel system outperformed competing mechanical thrombectomy devices...
A call to arms: Recruit physicians with endovascular skills to treat...
“There are simply not enough trained specialists to treat stroke patients in the US. We need to recruit physicians with endovascular skills—interventional cardiologists,...
Leading interventional radiology societies call for expanded stroke training
In a joint, global position statement, the Society of Interventional Radiology (SIR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE) and the Interventional Radiology...
Penumbra launches JET 7 and Penumbra JET D in USA
Penumbra has announced US commercial availability of the Penumbra JET 7 and Penumbra JET D Reperfusion Catheters powered by the Penumbra ENGINE aspiration source....
Strike now to perform stroke thrombectomy
Should interventional radiologists up-skill to perform emergency stroke thrombectomy and help deliver stroke services? And if they did, would the increase in the number...
New products showcased at SIR 2018
There were several devices in the New Product Showcase at the Society of Interventional Radiology's Annual Scientific Meeting in Los Angeles, USA. Among these...
New ischaemic stroke guidelines widen mechanical thrombectomy window to 24 hours...
The American Heart Association and American Stroke Association released updated ischaemic stroke guidelines that were published in Stroke, and released during the International Stroke...
New UK guidance on training IR to speed up roll out...
Emergency mechanical thrombectomy dramatically increases a stroke patient’s chance of a full recovery. The UK does not have enough skilled doctors to provide the procedure....
ASTER trial finds “no increased rate of revascularisation” with stent retriever...
The ASTER randomised clinical trial that examined the effect of endovascular contact aspiration vs. stent retriever on revascularisation in patients with acute ischaemic stroke...
ASTER results support use of Penumbra aspiration as frontline thrombectomy therapy...
Penumbra has announced the presentation of the results of the ASTER trial, the first independent, prospective, randomised trial comparing the use of Penumbra’s aspiration...