Tag: Penumbra
Penumbra unveils latest iteration of thrombectomy system for venous thromboembolism
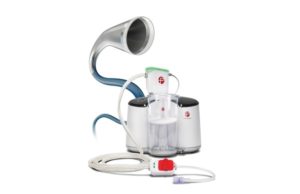
Penumbra has launched the Lightning Flash 3.0 computer-assisted vacuum thrombectomy (CAVT) system, a development that includes "significant upgrades" to the venous thromboembolism (VTE) platform,...
Boston Scientific announces agreement to acquire Penumbra
Boston Scientific and Penumbra today announced the companies have entered into a definitive agreement under which Boston Scientific will acquire Penumbra in a cash...
Penumbra receives CE mark for Lightning Bolt 12 and Lightning Bolt...
Penumbra recently announced that it has secured CE-mark approval for the Lightning Bolt 12 and Lightning Bolt 6X with TraX, which the company notes...
Penumbra receives CE mark for Lightning Bolt 12 and Lightning Bolt...
Penumbra recently announced that it has secured CE-mark approval for the Lightning Bolt 12 and Lightning Bolt 6X with TraX, which the company notes...
CAVT with anticoagulation significantly improves functional outcomes in PE patients, RCT...
Mechanical thrombectomy by means of computer-assisted vacuum thrombectomy (CAVT) using the 16Fr Lightning Flash system (Penumbra) along with anticoagulation for acute-to-intermediate pulmonary embolism (PE)...
STORM-PE finds mechanical thrombectomy superior to anticoagulation alone
The use of mechanical thrombectomy, specifically computer-assisted vacuum thrombectomy (CAVT) using the 16Fr Lightning Flash system (Penumbra), with anticoagulation achieves superior reduction in right...
Shruthi Narayan appointed president of Penumbra
Penumbra has today announced the promotion of Shruthi Narayan to president of the company, effective 1 September 2025.
A recent company press release states she will continue to...
Penumbra’s STORM-PE randomised trial completes enrolment
Penumbra has announced the completion of enrolment in the STORM-PE clinical trial.
The pivotal, prospective, multicentre randomised controlled trial enrolled 100 patients to evaluate computer...
New THRIVE data on Penumbra’s CAVT technology for acute limb ischaemia...
Recent THRIVE study data show that Penumbra’s computer-assisted vacuum thrombectomy (CAVT) technology not only has the potential to improve outcomes for lower extremity acute...
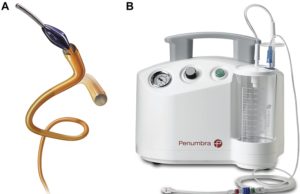
Penumbra receives US FDA clearance for Ruby XL system
Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of the Ruby XL system.
The Ruby XL system is designed to...
STRIKE-PE interim analysis shows benefit for acute PE patients with syncope
A STRIKE-PE interim analysis of clinical outcomes of acute pulmonary embolism (PE) patients with or without syncope—an indicator of compromised cardiac output and so...
Penumbra announces completion of enrolment in THUNDER IDE study
Penumbra has announced the completion of enrolment in its THUNDER investigational device exemption (IDE) clinical study for patients with acute ischaemic stroke.
THUNDER is evaluating...
Penumbra receives CE mark for Lightning Flash 2.0 and Lightning Bolt...
Penumbra today announced it has secured CE mark in Europe for two computer-assisted vacuum thrombectomy (CAVT) technologies—Lightning Flash 2.0 and Lightning Bolt 7.
“Based on...
12-month STRIDE data show high limb salvage rate and quality of...
One-year limb salvage and quality of life (QoL) data from the STRIDE study, showing that frontline use of Penumbra’s Indigo system for patients with...
Penumbra announces European launch of neuro access catheters
Penumbra has announced that it has received CE mark approval and so has initiated the European launch of BMX81 and BMX96, devices which are...
Penumbra launches Midway intermediate catheters and expands European stroke portfolio
Today, Penumbra announced the launch of its Midway 43 and Midway 62 delivery catheters, which are designed to provide ideal tracking and a stable...
Penumbra announces FDA clearance of Lightning Flash 2 for the treatment...
Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of Lightning Flash 2, the next generation computer assisted vacuum thrombectomy (CAVT)...
New data show Penumbra’s Indigo aspiration system used in a single...
During the Society of Interventional Radiology (SIR) 2024 annual meeting (23–28 March, Salt Lake City, USA), newly presented data from a subgroup analysis of...
EMBOLIZE pelvic venous disease study launched at SIR 2024
The Society of Interventional Radiology (SIR) Foundation, The VIVA Foundation and Penumbra, today announced the launch of the EMBOLIZE trial, a first-of-its-kind prospective, randomised...
Computer-assisted vacuum thrombectomy (CAVT) technology broadens patient applicability for complex disease...
NOTE: This video is ONLY available to watch in selected countries and geographies
Consultant interventional radiologist, Andrew Wigham (Oxford, UK) examines his experience with...
First patient enrolled in STORM-PE RCT evaluating Penumbra’s Lightning Flash for...
Penumbra today announced that the first patient has been enrolled in STORM-PE, a prospective, multicentre, randomised controlled trial (RCT) evaluating anticoagulation alone versus anticoagulation...
Interventional News Issue 92—November 2023
Interventional News 92 Highlights:
International IR voices map the path to primary specialty
Interventional News welcomes new editor-in-chief Robert Morgan
Profile: Jonathan Moss
CIRSE: Top...
Late breaking STRIKE-PE data shows improved outcomes for pulmonary embolism patients
Penumbra, a global healthcare company focused on innovative therapies, announced the latest STRIKE-PE data evaluating Penumbra’s Indigo aspiration system with Lightning. The results show...
Penumbra expands computer-aided thrombectomy offering with Lightning Bolt 7 launch
Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of Lightning Bolt 7, which the company claims is the most...
Penumbra launches new RED 43 reperfusion catheter for mechanical thrombectomy
Penumbra recently announced the launch of the latest addition to its RED family of catheter devices for mechanical thrombectomy, the RED 43 reperfusion catheter.
According...
Interventional News’ top 10 most popular stories of January 2023
Interventional News’ most popular stories in the opening month of 2023 included news of first enrolments in clinical studies of peripheral arterial disease (PAD)...
Penumbra launches Lightning Flash mechanical thrombectomy system
Today, Penumbra announced the US Food and Drug Administration (FDA) clearance and launch of its Lightning Flash mechanical thrombectomy system.
"Lightning Flash features Penumbra's novel...
Penumbra announces European launch of RED reperfusion catheters for stroke care
Penumbra announced today that its RED reperfusion catheters have secured a CE mark and are now commercially available in Europe. The catheters are part of...
Penumbra announces the European launch of the Indigo system with Lightning...
Penumbra has announced that its Indigo aspiration system with Lightning 7 and Lightning 12 have secured CE mark and are now commercially available in...
Penumbra announces US commercial availability of Indigo system Lightning 7
Penumbra today announced US commercial availability of the Indigo system Lightning 7. Lightning 7 expands Penumbra’s offering of the Indigo aspiration system with Intelligent...
EXTRACT-PE trial data confirm Indigo Aspiration system is safe and effective...
Data from the EXRACT-PE trial, published online first in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions, found that the Indigo...
Penumbra’s newest generation of Indigo aspiration system receives FDA clearance for...
Penumbra today announced US Food and Drug Administration (FDA) 510(k) clearance for expanded indication of the latest iteration of the Indigo aspiration system, Lightning...
Penumbra augments vascular franchise with latest Indigo System launch and newly...
The Indigo System Lightning 12 (Penumbra) is now commercially available in the USA, the company has revealed. In addition, Penumbra has announced the appointment...
VIVA 2019: Penumbra Indigo Aspiration system IDE trial for acute PE...
Penumbra today announced that the EXTRACT-PE trial successfully met the primary endpoints, demonstrating the safety and efficacy of the Indigo Aspiration system for aspiration...
Penumbra announces acquisition of controlling interest in MVI health
Penumbra, a global healthcare company focused on innovative therapies, has announced it has closed on the acquisition of a controlling interest in its joint...
Penumbra launches JET 7 and Penumbra JET D in USA
Penumbra has announced US commercial availability of the Penumbra JET 7 and Penumbra JET D Reperfusion Catheters powered by the Penumbra ENGINE aspiration source....
“Aspiration first” approach with Penumbra system demonstrates favourable outcomes for acute...
Penumbra announced results of the company-sponsored PROMISE study, demonstrating real-world safety and efficacy of the Penumbra system with ACE 68 and ACE64 reperfusion catheters...
Penumbra announces formation of virtual reality joint venture with Sixense Enterprises
Penumbra has announced that it has entered into a joint venture called MVI Health, for the purpose of exploring healthcare applications of virtual reality...
ASTER trial finds “no increased rate of revascularisation” with stent retriever...
The ASTER randomised clinical trial that examined the effect of endovascular contact aspiration vs. stent retriever on revascularisation in patients with acute ischaemic stroke...
ASTER results support use of Penumbra aspiration as frontline thrombectomy therapy...
Penumbra has announced the presentation of the results of the ASTER trial, the first independent, prospective, randomised trial comparing the use of Penumbra’s aspiration...