Tag: IRE
PRESERVE IRE trial demonstrates safety and efficacy in intermediate-risk prostate cancer
AngioDynamics has today announced the publication of results from the PRESERVE study, which assessed the safety and effectiveness of irreversible electroporation (IRE) with the...
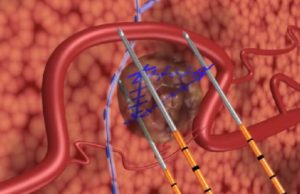
COLDFIRE 2: IRE is safe, effective in patients unsuitable for thermal...
Irreversible electroporation (IRE) is an effective and relatively safe treatment for colorectal liver metastases 5cm or smaller that are deemed unsuitable for partial hepatectomy,...
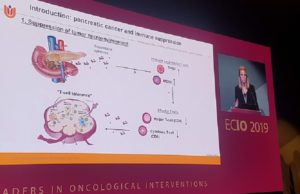
CROSSFIRE trial: IRE induces a more immune-permissive environment in pancreatic cancer...
Irreversible electroporation (IRE) significantly induces a window of reduced immune suppression two weeks post treatment, allowing the activation of effector T cells that seem...
Preliminary results indicate IRE stimulates the immune response to improve survival...
Hester Scheffer (Amsterdam, The Netherlands) advocates for the use of combined irreversible electroporation (IRE) and other immune stimulatory therapies to achieve greater overall survival...
AngioDynamics receives FDA approval to initiate NanoKnife DIRECT clinical study for...
The US Food and Drug Administration (FDA) have approved AngioDynamic’s investigational device exemption (IDE) application for NanoKnife Irreversible Electroporation “Direct IRE Cancer Treatment” clinical...
Physicians call for further work on IRE in pancreatic cancer
Further research into the use of percutaneous irreversible electroporation (IRE) in the treatment of pancreatic cancer is essential, according to Govindarajan Narayanan, chief of...
Irreversible electroporation has “potential to become new standard of care” in...
A new study presented at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE 2016, 10–14 September, Barcelona, Spain) meeting finds that the treatment...