Tag: Boston Scientific
Boston Scientific announces agreement to acquire Penumbra
Boston Scientific and Penumbra today announced the companies have entered into a definitive agreement under which Boston Scientific will acquire Penumbra in a cash...
Boston Scientific recalls Carotid Wallstent Monorail devices over “manufacturing defect”
Boston Scientific has recalled its Carotid Wallstent Monorail endoprosthesis owing to a “manufacturing defect” that has led to devices having an inner lumen that...
Real-world data show Cook’s Zilver PTX leads to lower rates of...
Cook Medical has announced that its Zilver PTX drug-eluting stent (DES) has lower rates of in-stent occlusions among patients with restenosis at three years than the Eluvia DES (Boston Scientific), according to...
Boston Scientific to acquire renal denervation technology developer SoniVie
Boston Scientific has entered into a definitive agreement to acquire SoniVie Ltd, a privately held medical device company that has developed the TIVUS intravascular...
Boston Scientific to acquire IVL developer Bolt Medical
Boston Scientific has announced it has entered into a definitive agreement to acquire Bolt Medical, the developer of an intravascular lithotripsy (IVL) advanced laser-based...
Kathleen Van Vlierberghe appointed vice president of Peripheral Interventions for Boston...
Boston Scientific today announced the appointment of Kathleen Van Vlierberghe as the new vice president for the Peripheral Interventions division in Europe, Middle East and...
Boston Scientific announces acquisition of Intera Oncology
Boston Scientific has announced it has entered into a definitive agreement to acquire Intera Oncology.
Intera Oncology’s Intera 3000 hepatic artery infusion pump has...
Real-world registry shows sustained efficacy for Eluvia DES in the SFA
This advertorial is sponsored by Boston Scientific.
During a satellite symposium session dedicated to real cases and trial outcomes which was held at the Cardiovascular...
Boston Scientific announces agreement to acquire Silk Road Medical
Boston Scientific has announced that it has entered into a definitive agreement to acquire Silk Road Medical, a medical device company involved in producing...
REAL-PE demonstrates statistically significant lower major bleeding rates with Ekos system...
Data from the REAL-PE study were presented this week at TCT 2023 (23–26 October, San Francisco, USA) demonstrating that patients treated for pulmonary embolism...
Boston Scientific receives 510(k) clearance for expanded cryoablation system
Boston Scientific has received 510(k) clearance for expanded indication of the Visual Ice cryoablation system. The clearance grants this device an indication expansion to...
Boston Scientific announces position on FDA update about use of paclitaxel-coated...
Following yesterday's news that the US Food and Drug Administration (FDA) has changed its stance on the use of paclitaxel-coated devices to treat peripheral...
Boston Scientific receives 510k clearance for Embold soft and packing coils
Boston Scientific has received US Food and Drug Administration (FDA) 510(k) clearance for its Embold soft and packing coils, the company has announced in...
Real-world data consistent with RCTs in highlighting the efficacy of drug-eluting...
This advertorial is sponsored by Boston Scientific.
During a recent satellite symposium, which took place at the 2022 Cardiovascular Interventional Radiological Society of Europe (CIRSE)...
Interventional News’ top 10 most popular stories of December 2022
Interventional News’ most popular stories as 2022 came to a close included news of a US Food and Drug Administration clearance for a new...
Interventional News’ top 10 most popular stories in 2022
Interventional News covered a whole range of the specialty's news during 2022, but readers' attention was most piqued by acquisitions (Boston Scientific's of Obsidio...
Boston Scientific announces partial acquisition offer for majority stake of Acotec...
Boston Scientific has announced that it will make a partial offer to acquire a majority stake, up to a maximum of 65%, of shares...
Truveta announces collaboration with Boston Scientific to advance post-procedure patient insights...
Truveta announced a strategic collaborative agreement with Boston Scientific to improve long-term patient care and gain insights into healthcare disparities. Through this collaboration, Boston...
Boston Scientific announces acquisition of Obsidio
Boston Scientific has announced the acquisition of Obsidio, a privately held company that has developed the Gel Embolic Material (GEM) technology used for embolization...
Boston Scientific announces agreement to purchase majority stake of M.I.Tech from...
M.I.Tech is the creator of the HANAROSTENT technology, a family of conformable, non-vascular, self-expanding metal stents, which have been distributed by Boston Scientific in...
Interventional News’ top 10 most popular stories of May 2022
May's top 10 stories on Interventional News include a report of long-term data on the safety of femoropopliteal endovascular treatment with paclitaxel-coated devices, FDA clearance of...
Boston Scientific Receives FDA Clearance for the EMBOLD Fibered Detachable Coil
Boston Scientific has received US Food and Drug Administration (FDA) 510(k) clearance for the EMBOLD Fibered Detachable Coil, which is indicated to obstruct or...
Evidence in the SFA: DES ‘will be the standard of care’...
NOTE: This video is ONLY available to watch in selected countries and geographies
Marianne Brodmann (Medical University of Graz, Division of Angiology, Austria) talks...
Evidence in the SFA: Eluvia has a “significantly better” patency rate...
NOTE: This video is ONLY available to watch in selected countries and geographies
Gunnar Tepe (interventional radiologist, Germany) discusses the results and subsequent impact...
Evidence in the SFA: Considering the data when making decisions for...
NOTE: This video is ONLY available to watch in selected countries and geographies
Koen Deloose (vascular surgeon, Belgium) looks at the evidence needed in order...
Time matters in the trauma setting
NOTE: This video is ONLY available to watch in selected countries and geographies
With trauma comes the challenge of not knowing what a patient is...
EMINENT one-year results show “superior” primary patency rate for Eluvia DES...
One-year results presented at Vascular Interventional Advances (VIVA) 2021 (5–7 October, Las Vegas, USA) from the EMINENT trial demonstrated the superiority of the Eluvia...
Two-year Ranger DCB data demonstrate “sustained, high rate” of device efficacy
Patients treated with the Ranger drug-coated balloon (DCB; Boston Scientific) sustained “improved primary patency” with fewer reinterventions than those treated with uncoated devices, two-year...
Positive data for the EkoSonic endovascular system presented at VIVA 2021
Boston Scientific announced positive results for the EkoSonic endovascular system (EKOS system) during a late-breaking clinical trial presentation at Vascular Interventional Advances (VIVA) 2021...
Late-breaking data indicate improved progression-free survival following TheraSphere treatment
Late-breaking trial data has demonstrated improved progression-free survival in patients with metastatic colorectal cancer after treatment with TheraSphere Y-90 Glass Microspheres (Boston Scientific).
Findings from...
Boston Scientific initiates randomised controlled trial for the EkoSonic endovascular system
Boston Scientific has commenced enrolment in the HI-PEITHO clinical trial, a collaborative research study with the Pulmonary Embolism Response Team (PERT) Consortium and the...
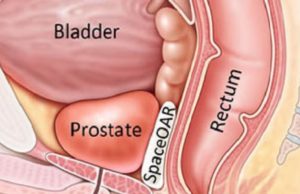
Boston Scientific announces European launch of SpaceOAR Vue Hydrogel
Boston Scientific has initiated the European launch of its SpaceOAR Vue Hydrogel, which is designed to create a temporary space between the prostate and the...
Trialists’ insights on the 2-year results from the COMPARE trial
NOTE: This video is ONLY available to watch in selected countries and geographies
Sabine Steiner (Leipzig, Germany) sits down with Michael Jaff, the CMO...
Boston Scientific receives FDA approval for TheraSphere Y-90 glass microspheres
The US Food and Drug Administration (FDA) have granted approval of the TheraSphere Y-90 glass microspheres (Boston Scientific), developed for the treatment of patients...
NICE recommends selective internal radiation therapies for the treatment of hepatocellular...
The National Institute for Health and Care Excellence (NICE) has issued guidance recommending the use of selective internal radiation therapy (SIRT) for the treatment...
New trial investigating combination therapy of radioembolization and immunotherapy in HCC...
The Society of Interventional Oncology (SIO) and Boston Scientific have announced a new multicentre, prospective, randomised trial to study combination therapy of radioembolization and...
Boston Scientific receives FDA approval for the Ranger DCB
Boston Scientific announced it has received US Food and Drug Administration (FDA) approval of the Ranger drug-coated balloon (DCB), developed for the treatment of...
Calls in Germany for reimbursement as analysis shows drug-eluting technology is...
Stefan Müller-Hülsbeck (Ev Luth Diakonissenanstalt zu Flensburg, Flensburg, Germany) presented attendees of the online meeting of the Cardiovascular and Interventional Radiological Society of Europe...
CMS grants additional reimbursement for the Eluvia drug-eluting vascular stent system
Boston Scientific announced that the US Centers for Medicare and Medicaid Services (CMS) granted a new technology add-on payment (NTAP) for the Eluvia drug-eluting...
Endovascular intervention for the treatment of femoropopliteal lesions: ELUVIA is safe...
This advertorial is sponsored by Boston Scientific.
Boston Scientific leads the largest clinical programme investigating drug-eluting therapy for the treatment of peripheral arterial disease (PAD),...
VIVA 2019: Boston Scientific announces positive data for the Ranger DCB...
Boston Scientific today announced positive data for two devices within the peripheral drug-eluting product portfolio during separate late-breaking clinical trial presentations at the 2019...
“Collaboration and innovation” is at core of Michael Jaff’s new role...
Boston Scientific recently announced that vascular medicine specialist Michael R Jaff (Newton-Wellesley Hospital, Newton, USA) will be joining the company as vice president, clinical...
Michael Jaff joins Boston Scientific to drive innovation in peripheral technologies
Michael R Jaff is to be vice president, clinical affairs, innovation and technology, peripheral interventions of Boston Scientific by January 2020. In this role,...
CIRSE 2019: Hear the latest from clinical trials comparing paclitaxel technologies...
NOTE: ONLY intended for healthcare professionals outside of the USA and France.
Zoom in on a tête-à-tete between Marianne Brodmann (Graz, Austria) and Giovanni Torsello...
Boston Scientific closes acquisition of BTG
Boston Scientific has announced the completion of its acquisition of BTG, pursuant to the previously announced scheme of arrangement. BTG develops and commercialises products...
Boston Scientific signs medical technology alliance with Mayo Clinic
Medical devices manufacturer Boston Scientific has partnered with Mayo Clinic, a non-profit academic medical centre, to launch an accelerator focused on medical solutions for...
Varian Medical acquires two Boston Scientific product lines
Radiation therapy firm Varian Medical Systems has signed an agreement to acquire Boston Scientific's portfolio of drug-loadable microsphere and bland embolic bead products designed...
Only Eluvia Uses a Polymer for Controlled, Targeted Delivery of the...
Eluvia DES is the only technology that uses polymer-based drug delivery to treat Peripheral Artery Disease (PAD). Its polymer ensures that the majority of...
Eluvia drug-eluting stent continues to demonstrate positive outcomes in IMPERIAL trial...
Boston Scientific has announced results from sub-analyses of the IMPERIAL clinical trial for the Eluvia Drug-Eluting Vascular Stent System. Data demonstrated that the efficacy...
Boston Scientific launches new stent for venous obstructive disease
Boston Scientific has won FDA approval for its Vici Venous Stent System for the treatment of iliofemoral venous obstructive disease.
The device received CE mark...
Hear the latest thoughts on paclitaxel from global experts: Their current...
Following the latest information presented at the Charing Cross (CX) International Symposium related to the meta-analysis of paclitaxel-coated devices (Katsanos et al), hear from a...
Medtronic and Boston Scientific stand by paclitaxel devices despite Katsanos’ critical...
Speaking to investors at the JP Morgan healthcare conference in San Francisco, executives from Medtronic and Boston Scientific said their data do not show...
Boston Scientific announces recommended offer to acquire BTG
Boston Scientific has announced it has reached an agreement on the terms of a recommended offer to acquire BTG, a company headquartered in the...
Positive outcomes for Eluvia stent in long lesion IMPERIAL sub-study
Clinical outcomes from the IMPERIAL Long Lesion Sub-study were presented at VIVA (5–8 November, Las Vegas, USA) demonstrating that the Eluvia drug-eluting vascular stent...
Eluvia outperforms Zilver PTX in IMPERIAL 12-month results
The Eluvia drug-eluting vascular stent system (Boston Scientific) shows superior primary patency compared to the Zilver PTX drug-eluting stent (Cook Medical), concludes the 12-month...
Boston Scientific announces agreement to acquire Augmenix
Boston Scientific has announced that it has entered into a definitive agreement to acquire Augmenix, a privately-held company which has developed and commercialised the...
Boston Scientific announces agreement to acquire Veniti
Boston Scientific has signed an agreement to acquire Veniti, a privately-held company in Fremont, USA which developed and commercialised the Vici venous stent system for...
Explore how to enhance your ability to access challenging treatment sites
This content is for distribution within USA only.
The Direxion™ Microcatheter, with its unique shaft design, offers you unrivaled torqueability for improved maneuverability. Further enhancing...
Our guidewire design revolutionizes the ability to access even the most...
This content is for distribution within USA only.
As you pursue new and innovative procedures within more complex anatomy, you need precision and control like...
First data from head-to-head comparison of Ranger and IN.PACT drug-coated balloons...
Early data from the first randomised controlled trial to compare two drug-coated balloons (DCBs) suggest that the primary patency obtained with the Ranger DCB...
Eluvia paclitaxel-eluting stent shows long-term freedom from revascularisation at three years
Boston Scientific has announced the three-year results from the MAJESTIC trial for the Eluvia paclitaxel-eluting vascular stent system at the Cardiovascular and Interventional Radiological...