Tag: Angiodynamics
AngioDynamics expands European indications for NanoKnife system
AngioDynamics has expanded indications across Europe for its NanoKnife system to include soft tissue ablation for tumours in the liver, kidney, prostate and pancreas,...
First patient in UK treated with new laser catheter for PAD
University Hospitals Plymouth (UHP) NHS Trust has become the first centre in the UK to treat patients with new laser catheter technology for vascular...
First patient enrolled in AngioDynamics’ AMBITION BTK trial for CLTI
AngioDynamics today announced enrolment of the first patient in AMBITION BTK, a randomised study of the Auryon atherectomy system in patients with below-the-knee chronic...
PRESERVE IRE trial demonstrates safety and efficacy in intermediate-risk prostate cancer
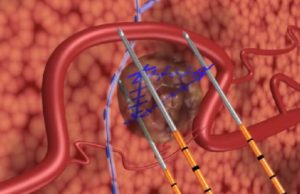
AngioDynamics has today announced the publication of results from the PRESERVE study, which assessed the safety and effectiveness of irreversible electroporation (IRE) with the...
First patient enrolled in RECOVER-AV trial of AlphaVac F18 system for...
AngioDynamics has announced the first patient has been enrolled in the RECOVER-AV clinical trial, a prospective, multicentre, international, single-arm study evaluating the AlphaVac multipurpose...
APEX-AV trial results of AlphaVac PE system published in JSCAI
AngioDynamics has announced the publication of the results from the Acute Pulmonary Embolism Extraction Trial with the AlphaVac system—APEX-AV—in the Journal of the Society...
AngioDynamics receives FDA clearance for prostate tissue ablation system
AngioDynamics has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for the NanoKnife system for prostate tissue ablation.
The company received clearance...
AngioDynamics announces CE mark approval in Europe for the Auryon system
AngioDynamics today announced European CE mark approval of the Auryon atherectomy system, designed for the treatment of peripheral arterial disease (PAD), including chronic limb-threatening...
AngioDynamics announces CE mark approval for AlphaVac system
AngioDynamics, a leading medical technology company focused on restoring healthy blood flow in the body’s vascular system, has announced European CE mark approval of...
AngioDynamics receives 510(k) clearance for AlphaVac system in treatment of PE
AngioDynamics, a leading and transformative medical technology company focused on restoring healthy blood flow in the body’s vascular system, expanding cancer treatment options and...
AngioDynamics announces US FDA 510(k) clearance of Auryon XL radial access...
AngioDynamics today announced that the US Food and Drug Administration (FDA) has cleared the Auryon XL catheter, a 225cm radial access catheter, for use with...
AngioDynamics completes enrolment for PRESERVE clinical study
AngioDynamics has announced the completion of enrolment and final treatment in its pivotal study of the NanoKnife system for ablation of prostate tissue in...
AngioDynamics announces FDA clearance of expanded indications for Auryon atherectomy system
AngioDynamics recently announced that it has received US Food and Drug Administration (FDA) 510(k) clearance of an expanded indication for the Auryon atherectomy system...
AngioDynamics receives 510(k) clearance for AlphaVac mechanical thrombectomy system
AngioDynamics recently announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the AlphaVac mechanical thrombectomy system.
According to...
“A new look at laser”: Positive initial data presented at ISET...
The Auryon atherectomy system (AngioDynamics) “represents an exciting new technology”, John Rundback (Advanced Interventional and Vascular Services, LLP, Teaneck, USA) opined at the International...
AngioDynamics announces presentation of positive safety, efficacy results from RAPID outcomes...
AngioDynamics has announced the safety and efficacy results from the RAPID (Registry of AngioVac system procedures in detail) database. Results were shared by principal...
First patient enrolled in PATHFINDER I registry examining Auryon atherectomy system
AngioDynamics has announced the enrolment of the first patient in the PATHFINDER I postmarket registry.
The PATHFINDER I registry is a pilot study to evaluate...
PATHFINDER I launches registry to evaluate performance and clinical outcomes of...
AngioDynamics has announced the launch of the PATHFINDER I: Post-Market Registry (PATHFINDER I-Registry, EX-PAD-05), a pilot study to evaluate the safety and efficacy of...
AngioDynamics receives FDA approval to initiate NanoKnife DIRECT clinical study for...
The US Food and Drug Administration (FDA) have approved AngioDynamic’s investigational device exemption (IDE) application for NanoKnife Irreversible Electroporation “Direct IRE Cancer Treatment” clinical...
AngioDynamics provides update on vascular access business
AngioDynamics has provided a corporate update and acknowledged a recent, favourable US District Court ruling that impacts its vascular access business.
A press release states...
Solero microwave ablation system gets CE mark
Angiodynamics has announced it has received CE mark certification for the Solero microwave tissue ablation system. Solero and its accessories are indicated for the...