Shape Memory Medical recently announced that it has received approval from the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) to market its Impede-FX embolisation plug in Japan. Cosmotec championed the approval process in Japan and is Shape Memory Medical’s distribution partner for its peripheral embolisation products.
The Impede-FX embolisation plug is indicated for use in Japan as an adjunct to devices such as stents, coils, and plugs, and the Impede embolisation plug which previously received PMDA approval in 2019. Impede-FX is percutaneously deployed in an artery or vein for obstructing blood flow in abnormal blood vessels like arteriovenous malformation and fistula, aneurysms, bleeding by traumatic vascular injury, tumours, etc. except for intracranial and cardiac vessels. Impede-FX is available in three sizes, with the maximum device having an expanded diameter of 12mm.
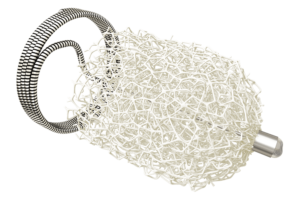
Impede embolisation plugs feature the proprietary Shape Memory polymer technology, a porous, embolic scaffold that is crimped for catheter delivery and self-expands upon contact with blood for rapid conversion to organised thrombus. Preclinical and clinical studies have shown that Shape Memory polymer offers effective and predictable space filling compared to traditional coils and plugs, stable clot formation for reduced intradevice recanalisation, and progressive healing as the material biodegrades. To date, nearly 600 patients have been treated worldwide with the Impede embolisation plug family of devices.











