Surmodics has announced that its Pounce thrombectomy system achieved 100% technical success in 20 first-in-human (FIH) procedures. The FIH data were presented by Gary Ansel (Columbus, USA) at the 2022 Charing Cross (CX) Symposium (26–28 April, London, UK).
Surmodics has announced that its Pounce thrombectomy system achieved 100% technical success in 20 first-in-human (FIH) procedures. The FIH data were presented by Gary Ansel (Columbus, USA) at the 2022 Charing Cross (CX) Symposium (26–28 April, London, UK).
Technical success was defined as the removal of clot to restore blood flow without the use of an additional adjunct thrombectomy device. Ansel, the inventor of the Pounce thrombectomy system and a consultant for Surmodics, also revealed that 19 of the 20 procedures were able to avoid the use of thrombolytics in the target lesion. The FIH cases were performed across six US medical centres, with the first use of the US Food and Drug Administration (FDA)-cleared system occurring in June 2021.
“The Pounce thrombectomy system demonstrated technical success in these early clinical cases, showing an ability to quickly deal with a wide range of clot from soft to organised, including emboli in the peripheral arterial vasculature,” said Ansel. “This fully mechanical thrombectomy device has no power unit or other capital equipment requirement, making it easy to use and efficient for physicians to treat patients with complex peripheral arterial disease (PAD).”
Average procedure time in the FIH cases was 79.6 minutes and the average lesion length measured 109mm (range 5–300mm). Clinical presentation time ranged from one hour to eight months, with 30% of cases involving acute on chronic clot. Chronic clot (25%), acute clot (25%) and subacute (15%) was also removed. Five per cent of cases did not indicate clot classification. The superficial femoral artery was the most common vessel treated (50%) while 40% of the cases involved the popliteal artery.
“The Pounce thrombectomy system has the potential to revolutionise the treatment algorithm for arterial thrombectomy,” said Gary Maharaj, Surmodics CEO. “By providing peripheral interventionalists with an innovative, non-surgical tool for treating arterial thrombotic occlusions with a single session device designed to achieve an on-table result, this device will serve an important clinical need while advancing therapies for PAD.”
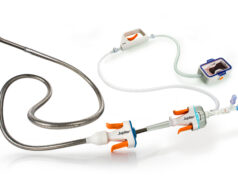
The Pounce thrombectomy device is comprised of three components: a 5Fr delivery catheter, a basket wire, and a funnel catheter. The basket wire is delivered distal to the location of the thrombus, deploying two nitinol self-expanding baskets. The baskets capture the clot and are retracted into a nitinol collection funnel. With the clot entrained, the system is withdrawn into a minimum 7Fr guide sheath through which the clot is withdrawn and removed from the body.