Orchestra BioMed, in partnership with Terumo Corporation, today announced that the company has secured breakthrough device designation by the US Food and Drug Administration (FDA) for its Virtue sirolimus-eluting balloon (SEB) in the treatment of below-the-knee (BTK) peripheral arterial disease.
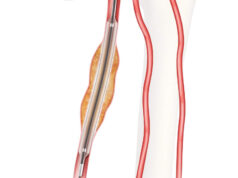
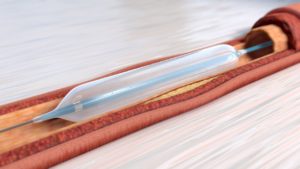
According to a press release, Virtue SEB is a novel, first-in-class drug/device combination product that delivers a sustained-release sirolimus formulation directly to the artery during balloon angioplasty without the need for a coating.
“Virtue SEB’s unique design enables delivery of sustained-release sirolimus during angioplasty without the need for coating or permanent implant. This highly differentiated design makes this product the ideal candidate for Breakthrough Device Designation in BTK peripheral artery disease,” says James P Zidar, clinical professor of medicine, UNC Health Systems, physician-in-chief, Heart & Vascular Corporate.
He continues: “Currently, there is a significant unmet need in the BTK stenosis treatment landscape. The presence of underlying comorbidities renders many patients unsuitable for bypass surgery. Angioplasty with plain balloons, which has been the default endovascular therapy for years, has a low success rate. Adding a proven anti-restenotic agent like sirolimus has the potential to enhance this treatment approach and drive better patient outcomes.”
“Our team is grateful that the FDA has recognised the potential value Virtue SEB can provide patients and physicians by granting this second Breakthrough Device Designation for an important arterial therapeutic indication,” said Darren R Sherman, president, chief operating officer and co-founder of Orchestra BioMed. “This designation will be critical as we continue to work with Terumo to accelerate Virtue SEB’s global clinical and regulatory program in both coronary and peripheral indications. In BTK disease, treatment with Virtue SEB has the potential to improve long-term outcomes and reduce periprocedural complications which can extend hospital stay and increase cost of treatment.”