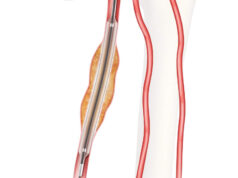
Results of the VIVO clinical study support the safety and effectiveness of Cook Medical’s recently FDA-cleared Zilver Vena venous stent for the treatment of symptomatic iliofemoral venous outflow obstruction, Anthony Comerota (Inova Fairfax Hospital, Alexandria, USA) revealed in a late-breaking data session at this year’s Vascular Interventional Advances conference (VIVA 2020; 6–8 November, virtual).
In addition, Comerota reported that VIVO study patients were “consistent with those in previous literature reports and reflective of a real-world population, including acute and chronic disease, and thrombotic and non-thrombotic lesions”.
Patients with symptomatic obstruction (CEAP clinical classification ≥3 or VCSS pain score ≥2) of one iliofemoral venous segment were included. The primary safety endpoint was 30-day freedom from major adverse events (MAE): procedural bleeding requiring transfusion, procedure- or device-related death, clinically-driven reintervention (reintervention with recurrent symptoms of venous outflow obstruction of the target lesion and a minimum lumen diameter [MLD] ≤50% of the immediate post-procedure MLD by venography), clinical migration, new symptomatic pulmonary embolism, or procedure-related perforation requiring open surgical repair or flow limiting dissection of the target vessel.
The primary effectiveness endpoint was 12-month rate of primary quantitative patency (intervention-free, MLD >50% of the immediate post-procedure MLD by venography). The secondary endpoint was the change in VCSS from baseline to 1 month and 12 months.
Comerota reported that 243 patients were enrolled (53±15 years; 70% female). Iliac vein compression by the iliac artery (n=191; 78.6%) was the primary indication for stent placement. The mean lesion length at baseline was 98.6±69.8mm. Technical success (ability to deliver and place stent in intended location) was achieved for 97.3% of stents. The 30-day freedom from MAE rate was 96.7%, exceeding the performance goal of 87% (95% confidence interval [CI]: 93.5–98.6%, p<0.0001). The 12-month primary quantitative patency rate was 89.9%, exceeding the performance goal of 76% (95% CI: 85.1–93.4%, p<0.0001). Clinical improvement was demonstrated by the improvement in VCSS from baseline: -3.0 (95% CI: -3.5 to -2.6, p<0.0001) at one month and -4.2 (95% CI: -4.7 to -3.7, p<0.0001) at 12 months.
Following Comerota’s presentation, panel member Kenneth Cavanaugh (US Food and Drug Administration [FDA] Office for Cardiovascular Devices, Silver Spring, USA) was interested in the potential value of adding a comparator into these studies now that there are other stents on the market.
Comerota responded by highlighting that certain factors would need to be taken into account in any head-to-head comparison of venous stents, including the potential for lesion length to vary widely. “If you were to choose an ideal lesion it would be short, it would be limited to the iliac segment, and it would be a stenosis, not an occlusion” he said, noting that in the VIVO study one-third of the patients had their stents extended to below the inguinal ligament, representing a “real challenge”.