To become a mainstay liquid embolic for peripheral use, an optimal product should be easy to use, have reproducible and controllable deep penetration for tumour and organ applications, be compatible with aqueous contrasts, drugs and standard microcatheters and not be prone to non-target embolization, writes Suvranu Ganguli, Boston, USA, on the topic of embolic agents that are just around the corner. embolics
Embolic particles and coils are the current mainstay of peripheral embolotherapy. Liquid embolics such as Onyx liquid embolic system (Medtronic) and TruFill (Cordis, now Cardinal Health) are
currently approved for neurological applications in the USA. These two liquid embolics are often used off label for peripheral applications, but they do have limitations with the inability to achieve diffuse distal embolization and challenges in delivery
technique. To become a mainstay liquid embolic for peripheral use, an optimal product should be easy to use, have reproducible and controllable deep penetration for tumour and organ applications, be safe from non-target embolization, and be compatible with aqueous contrasts, drugs and standard microcatheters. This is an overview of some liquid embolics in development, working to deliver this long sought after tool for interventional therapy.
Hydrogel Embolic System
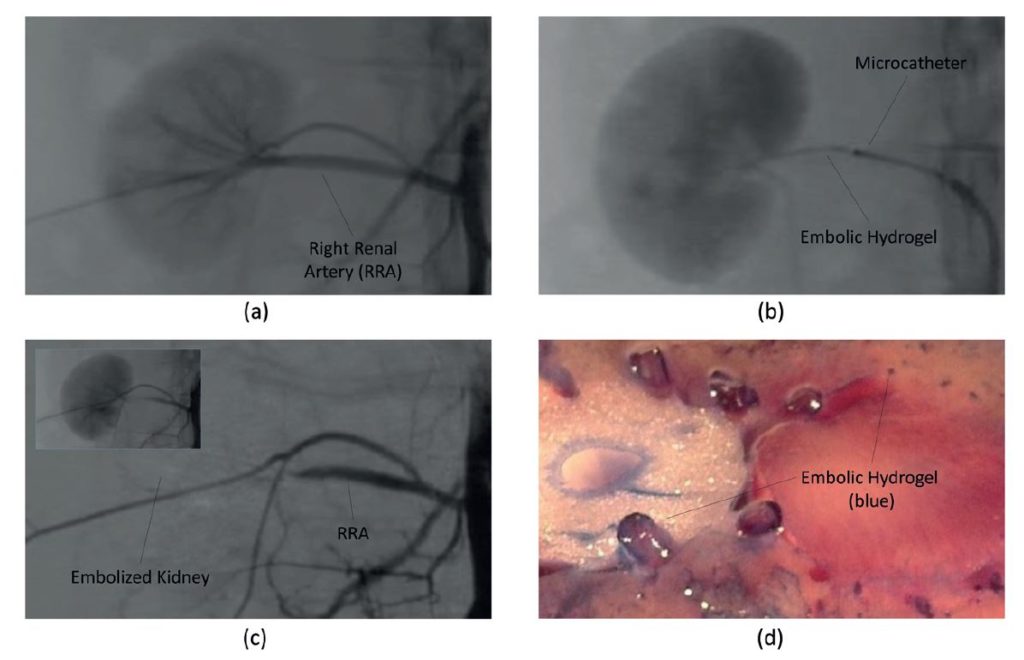
Instylla’s Hydrogel Embolic System (HES) is being designed for both the diffuse embolization of tumours and organs as well as specific segmental embolizations, such as in the setting of haemorrhage requiring proximal vessel occlusion. Instylla is the latest in the Incept family of companies that has been responsible for the development of more than a dozen innovative hydrogel based medical products used in millions of patients worldwide. The HES is a biocompatible, hydrophilic, hydrogel designed to offer controlled, safe and targeted embolization. HES is formed from precursors of functionalised polyethylene glycol that self react to form a hydrogel rapidly. Once delivered, the HES materials polymerise in-situ but with the location of embolization (distal or proximal) reliably controllable through simple adjustments in the rate and volume of delivery, much like techniques used with embolic particles. The HES is formulated to inhibit the gel from forming in non-target branching vessels, limiting non-target embolization. Since the polymerisation is self reacting and not based on precipitation (as in the case of Onyx or glues) the delivery procedure can be paused by the physician to provide an additional aspect of procedure control. The embolization is also blood and coagulation independent. These qualities support significantly reduced embolization procedure times but with safety and control. The resulting hydrogel is soft with a feel much like tissue, but physiologically stable and unaffected by natural thrombolytic processes that may cause recanalisation. Both small and large animal studies have demonstrated rapid and complete embolisation of diffuse vascular beds down to sub 10 microns as well as the ability to create discrete proximal vessel occlusions in a variety of vessel sizes, and in both cases avoiding nontarget embolization. The company is currently expanding its set of published in vitro and in vivo data and preparing for the first human clinical studies with the HES (Figure 1).
Shear-Thinning Biomaterial
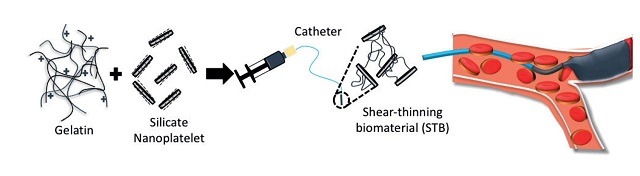
A team from Mayo Clinic Arizona and UCLA Bioengineering, USA, is developing a liquid embolic based on shear-thinning biomaterial (STB), a nanocomposite hydrogel containing gelatin and silicate nanoplatelets, to function as an embolic agent for endovascular embolization procedures (Figure 2). STBs are injectable through clinical catheters and needles and have haemostatic activity comparable to metallic coils, the current gold standard. In addition, STBs withstand physiological pressures without fragmentation or displacement in elastomeric channels in vitro, in explant vessels ex vivo and in large animal models. In vitro experiments also indicated that STB embolization did not rely on intrinsic thrombosis as coils did for occlusion, suggesting that the biomaterial may be suitable for use in patients on anticoagulation therapy or those with coagulopathy. Using CT imaging, the biomaterial was shown to fully occlude murine and porcine vasculature in vivo and remain at the site of injection without fragmentation or nontarget embolization.
Given the advantages of rapid delivery, in vivo stability, and independent occlusion that does not rely on intrinsic thrombosis, STBs offer an alternative gel-based embolic agent with translational potential for endovascular embolization. Furthermore, the STB does not generate artifact from imaging allowing visualisation of the embolized vasculature. STB as an embolic agent was the cover story in the November 2016 issue of Science Translational Medicine; research on STB is ongoing with two NIH R01 grants.
GPX
Fluidix Medical’s GPX, a liquid embolic, is based on biomaterials from sandcastle worms. These biomaterials are formulated into a colloidal-rich coacervate which when delivered into blood precipitates out the biomaterials to form the embolic. At high ionic strengths, the embolic coacervates are injectable fluids that can be delivered through long narrow microcatheters. At physiological ionic strength, the embolic coacervates transition into a nonflowing solid morphology. Transcatheter embolization of rabbit renal arteries demonstrated capillary level penetration, homogeneous occlusion, and 100% devascularisation of the kidney, without the embolic crossing into venous circulation. The benign waterborne composition and setting mechanism avoids many of the problems of current liquid embolics, and provides precise temporal and spatial control during endovascular embolization.
Silk Elastin Protein Polymer
Theratarget is developing a liquid embolic using a Silk Elastin protein Polymer (SELP). Theratarget was formed from research on SELP conducted at the University of Utah through the USTAR Initiative. The SELP material is a liquid at room temperature but at the internal human body temperature of 37°C, it transitions into a solid hydrogel capable of vessel occlusion. Since it is a single component system, it can be delivered using standard microcatheters. It is currently in development for chemoembolization with in vitro and animal model in vivo studies performed showing the feasibility of the product.
Suvranu “Shoey” Ganguli is associate chief, Interventional Radiology, Massachusetts General Hospital Harvard Medical School, Boston, USA. He is a consultant to Instylela, Boston Scientific, Medtronic and Sirtex.













