Endologix has shared in a press release that the first patients underwent percutaneous transmural arterial bypass (PTAB) using the Detour system since US Food and Drug Administration (FDA) approval of the system was granted. This marks the official start of its US targeted market release.
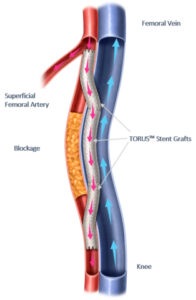
PTAB with the Detour system offers a disruptive, novel approach to treating complex peripheral arterial disease (PAD), enabling physicians to bypass lesions in the superficial femoral artery, by using stents routed through the femoral vein to restore blood flow to the leg. This approach is effective for patients with long lesions (20–46cm in length), those that have already undergone failed endovascular procedures, or those that may be suboptimal candidates for open surgical bypass.
For the rollout, Endologix collaborated with two healthcare systems known for clinical excellence. PTAB using the Detour system was first performed at Cleveland Clinic’s Sydell and Arnold Miller Family Heart, Vascular & Thoracic Institute (Cleveland, USA) and at Salinas Valley Health Medical Center (Salinas, USA).
“We are delighted to be able to introduce PTAB using the Detour system into clinical use,” said Matt Thompson, president, and CEO of Endologix. “The Detour system is a pivotal addition to our product portfolio, which now offers differentiated therapies for both abdominal aortic aneurysms and PAD. The successful introduction at these leading institutions underscores our commitment to continuing to innovate on behalf of patients. Broadening our therapeutic profile is a key achievement as we transform Endologix into a leading interventional vascular company.”










This is both exciting and progressive! Makes absolute sense especially when dealing with complex calcium and/or peripheral CTOs. I can’t wait to see patient outcomes.