Tag: peripheral
FastWave Medical announces successful 30-day first-in-human data on peripheral IVL technology
FastWave Medical has announced the 30-day results of its first-in-human (FIH) study using the company's differentiated peripheral intravascular lithotripsy (IVL) technology.
This was a...
Terumo Medical launches peripheral coil system in the USA
Terumo Medical, announces the launch of its new AZUR HydroPack peripheral coil system in the USA. The AZUR HydroPack coil system is a soft,...
Cardio Flow announces US FDA 510(k) clearance for its FreedomFlow orbital...
Cardio Flow recently announced it has received US Food and Drug Administration (FDA) 510(k) clearance for the company’s FreedomFlow orbital atherectomy peripheral platform.
The company...
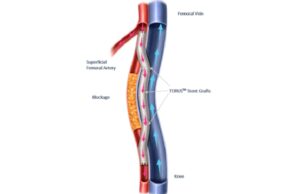
Endologix announces first patients treated with the Detour system
Endologix has shared in a press release that the first patients underwent percutaneous transmural arterial bypass (PTAB) using the Detour system since US Food...
Biotronik launches Oscar multifunctional peripheral catheter at LINC 2023
Biotronik today announced the limited release of its Oscar (One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter and start of promotional activities at this...
Getinge receives US FDA premarket approval for the iCast covered stent...
Getinge's iCast covered stent system has received premarket approval from the US Food and Drug Administration (FDA) for the treatment of patients with iliac...
Shockwave Medical announces US launch of new peripheral IVL catheter
Shockwave Medical today announced the full US commercial availability of the Shockwave L6 peripheral intravascular lithotripsy (IVL) catheter following clearance by the US Food...
Biotronik launches Oscar multifunctional peripheral catheter
Biotronik has announced the US Food and Drug Administration (FDA) 510(k) clearance and CE mark of its Oscar (One Solution: Cross. Adjust. Restore) multifunctional...
Interventional News’ top 10 most popular stories in 2022
Interventional News covered a whole range of the specialty's news during 2022, but readers' attention was most piqued by acquisitions (Boston Scientific's of Obsidio...
Chocolate Touch drug-coated angioplasty balloon for treatment of peripheral artery disease...
Genesis MedTech Group announced that the US Food and Drug Administration (FDA) has approved the Chocolate Touch drug-coated balloon (DCB) percutaneous transluminal angioplasty (PTA)...
Merit Medical launches the Prelude Roadster guide sheath
Merit Medical Systems has announced the US commercial release of the Prelude Roadster guide sheath. The Prelude Roadster is the newest addition to the...
Boston Scientific announces acquisition of Obsidio
Boston Scientific has announced the acquisition of Obsidio, a privately held company that has developed the Gel Embolic Material (GEM) technology used for embolization...
Selution SLR receives second FDA IDE approval
Selution SLR, MedAlliance's sirolimus-eluting balloon, has received conditional US Food and Drug Administration (FDA) investigational device exemption (IDE) approval to initiate its pivotal clinical...
New project set to develop nanoparticles with imaging and medicine-delivery capabilities...
A University of Texas at Arlington (UTA; Arlington, USA) bioengineer is leading a project that will develop biodegradable nanomaterials that will take pictures and...
Cardio Flow announces FDA clearance for FreedomFlow peripheral guidewire
Cardio Flow has announced it recently received US Food and Drug Administration (FDA) clearance for the company’s FreedomFlow peripheral guidewire.
According to a company press...
Interventional News Issue 86—June 2022 US Edition
Interventional News 86 Highlights:
Eyes on intravascular lithotripsy
The vascular biology behind endovascular therapies
Profile: Yasuaki Arai
CX: Transfemoral access takes centre stage at...
Interventional News Issue 86—June 2022 Edition
Interventional News 86 Highlights:
Eyes on intravascular lithotripsy
The vascular biology behind endovascular therapies
Profile: Yasuaki Arai
CX: Transfemoral access takes centre stage at...
S.M.A.R.T. Radianz vascular stent system approved for transradial use in the...
Cordis recently announced that the US Food and Drug Administration (FDA) has approved the S.M.A.R.T. Radianz vascular stent system, a self-expanding stent purposefully engineered...
CX returns to in-person format once more in the London spring
Charing Cross (CX) chair Roger Greenhalgh welcomes the vascular community to this year's symposium, due to be held 26–28 April in London, UK, and...
FDA clears 12 new XO Cross microcatheters
Transit Scientific has announced US Food and Drug Administration (FDA) clearance of new hydrophilic-coated XO Cross microcatheters for guidewire support, exchange, and contrast media...
Study provides new tool to assess amputation risk following popliteal vascular...
A large, multicentre cohort study provides a simple, practical method to effectively stratify patients preoperatively into low- and high-risk major amputation categories.
According to lead...
Biotronik expands range of peripheral introducer sheaths
Biotronik has announced the expansion of the Fortress reinforced introducer sheath line, which is now available in 7- and 8Fr-compatible sizes in the USA...
Terumo introduces Azur vascular plug and PG Pro peripheral microcatheter embolisation...
Terumo Medical Corporation has announced today the introduction of its Azur vascular plug. The addition to Terumo's embolisation portfolio is indicated for use to...
No increased mortality with paclitaxel use in Veterans Administration data out...
There was no increased risk of long‐term, all‐cause mortality associated with paclitaxel-coated device (PCD) use among patients undergoing femoropopliteal peripheral endovascular intervention within the...
Philips launches QuickClear mechanical thrombectomy system for blood clot removal in...
Royal Philips has announced the launch of the QuickClear mechanical thrombectomy system. The single-use system delivers an all-in-one aspiration pump and catheter to remove...
First patients enrolled in SUPERSURG study of Supera peripheral stent system
ID3 Medical Belgium has announced the first two enrolments in the SUPERSURG study to investigate the safety and efficacy of Abbott's Supera peripheral stent...
XableCath crossing catheters have received CE mark for peripheral use
XableCath has announced that its XableCath Crossing catheters have received CE mark for peripheral use. Its crossing devices will be available for sale in...
Terumo gets CE mark for Kanshas drug-coated balloon catheter for lower...
Terumo has announced receiving the CE mark for its Kanshas drug-coated balloon catheter used in the treatment of lower extremity peripheral arterial disease.
With...
First patients enrolled in Wing-IT investigational device exemption clinical trial
Reflow Medical announced that the first patients have been enrolled in a prospective, multicentre, non-randomised study intended to evaluate the ability of the Reflow...
New liquid embolic agents, Squidperi 34 and 34LD, get CE mark
Balt International announced that it has received CE mark for the Squidperi 34 and 34LD liquid embolic agents.
Squidperi is a non-adhesive liquid embolic agent...
Surmodics gets US FDA 510(K) clearance for Telemark support microcatheter
Surmodics has announced receiving US FDA 510(k) clearance for its Telemark 0.014” coronary and peripheral support microcatheter. The company is making this product available...
BTG receives US FDA 510(k) clearance for Ekos control unit 4.0
BTG has announced that US FDA 510(k) clearance has been granted to the Ekos control unit 4.0.
The Ekos system includes an ultrasonic device...
Medtronic receives US FDA clearance for TrailBlazer angled peripheral support catheter
Medtronic has announced that the US FDA has cleared the TrailBlazer angled support catheter for use in the peripheral vascular system. Support catheters such...
UK NICE publishes Medtech Innovation Briefing on Lutonix
The UK National Institute for Health and Care Excellence (NICE) has developed a Medtech Innovation Briefing (MIB) on the Lutonix drug-coated balloon (DCB) for...