During a late-breaking data session at this year’s Vascular Interventional Advances annual meeting (VIVA 2020; 6–8 November, virtual), Ehrin Armstrong (University of Colorado, Denver, USA) presented two-year outcomes from the DETOUR I trial for percutaneous femoropopliteal bypass. He reported a 96% clinical success rate, with 83% of patients at Rutherford class 0 at two years, which “shows excellent functional improvement in a patient population facing severely debilitating PAD [peripheral arterial disease]”.
The DETOUR I trial was designed to evaluate the safety and effectiveness of the Detour system for percutaneous femoropopliteal bypass, which recently received US Food and Drug Administration (FDA) breakthrough device designation.
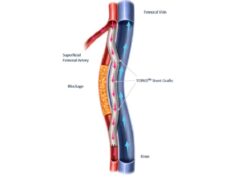
Using the novel PQ crossing device and a series of specially designed Torus stent grafts, the Detour procedure was created to route blood flow around severe lesions caused by complex peripheral arterial disease found in the superficial femoral artery. The procedure uses the femoral vein as a pathway for a Torus stent graft conduit and travels from the artery to the vein and back into the artery.
Now reporting two-year data, the trial enrolled a complex patient cohort with an average lesion length of 371mm; 96% of lesions had a confirmed chronic total occlusion, and 80% had evidence of moderate to severe calcification. At one and two years’ post-procedure, the study is reporting an 81% and 79% primary patency rate, respectively, as well as a rate of freedom from major adverse events of 83.7% and 82.1% at one and two years, respectively.
Armstrong also announced that, with FDA breakthrough designation and recent enrolment completion, DETOUR II is “on track to provide results earlier than expected”.