Ablation is entering a new era of increased precision and quantification, the interventional radiology (IR) community argues, with outcomes that match or surpass those of surgery. Whilst reaching equipoise with their surgical counterparts has been a goal for interventional radiologists since the advent of ablative treatments for cancer, some expert interventionalists claim that the increased precision of modern thermal ablation techniques, coupled with improvements in radiology mean physicians are often treating smaller volume tumours. This now positions the procedure as the “definitive” treatment for select patients in some cancers. Several clinical trials including the ACCLAIM, COLLISION, and COVER-ALL trials—are currently underway, and aim to bolster the evidence-base demonstrating favourable patient outcomes from thermal ablation.
“We are now talking about ablation 2.0,” past Society of Interventional Oncology (SIO) president Stephen Solomon (Memorial Sloan Kettering Cancer Center, New York, USA) says. “Ablation 1.0”, in his eyes, is the basic concept underpinning the minimally invasive procedure: put a needle in, use image guidance, burn or freeze the cancerous tissue to destroy it. “But ablation 2.0,” he explains, “means that we are focusing more on precision, on margins, and specifically the road to A0 [where all tumour cells are eradicated].”
When discussing soft and hardware advances in ablation, David Breen (University Hospital of Southampton, Southampton, UK) adopts the framework of “planning, execution, confirmation”. Planning uses preprocedural scans to map out probe positions and angles, along with estimation of the ablation zone—all the steps taken ahead of performing the ablation. Execution is the act of probe positioning, sometimes using guidance tools. The final stage, confirmation, involves determining the adequacy of treatment margins in order to verify complete ablation. “Of these three stages, confirmation and perhaps planning are the two that are beginning to enter a higher level of engineering maturity and reliability. Guidance tools, as they currently stand, must still be used with careful operator insight,” Breen explains.
The upcoming European Conference on Interventional Oncology (ECIO; 10–13 April, online) is hosting a session entitled “Different ways of killing cancer and why we need all of them”, which explores advances in ablation.
In a further sign of the times, several large companies are positioning themselves to buy smaller businesses operating in this space. In 2020, for example, imaging behemoth Siemens Healthineers acquired all shares of Varian, a radiotherapy company, which in 2019 itself acquired Endocare, a company specialising in cryoablation and microwave ablation, and Alicon, a provider of embolic therapy for liver cancer treatment.
Cascination is another company offering an over-arching solution for ablation, with products designed for imaging, planning, navigation, validation, treatment, and treatment verification. Breen believes this is the direction industry is taking, and represents a shift away from a more modular approach where one organisation may only offer products for part of this pathway.
Developing improved planning software
Several different groups around the world are working on developing planning software for ablation treatments with the goal of obtaining a predictable technical outcome. “In order to do this effectively,” current SIO president Matthew Callstrom (Mayo Clinic, Rochester, USA) explains, “it is necessary to have accurate registration software for all phases of the ablation, including planning, intraprocedural evaluation of device placement, and postprocedural measurement of the margins of the ablation. This approach will transition thermal ablation from a subjective technical endpoint to an objective technical endpoint. I think the exciting aspect of this is that many efforts are underway and will become more widely available to proceduralists.”
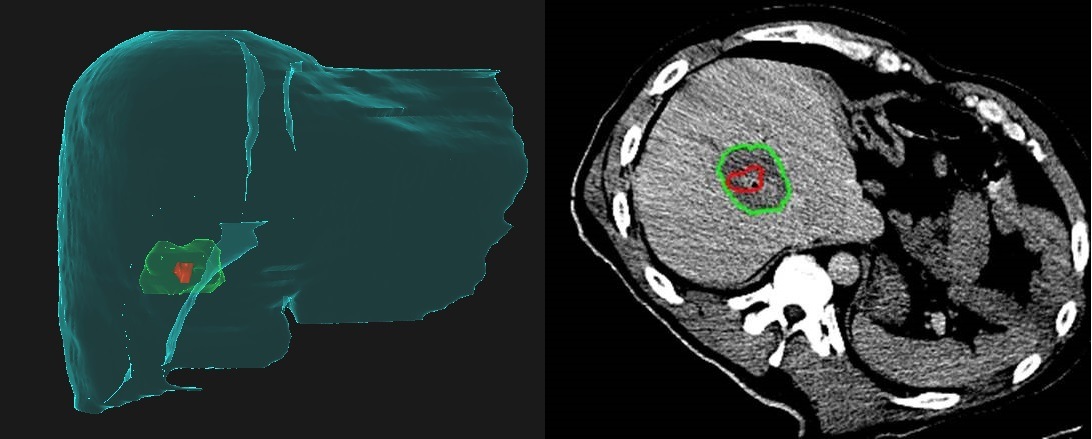
From the department of Interventional Radiology at the University of Texas MD Anderson Cancer Center (Houston, USA), Bruno Odisio tells Interventional News that he and imaging physicist Kristy Brock have received a National Institute of Health (NIH) grant (Academic-industry partnership–Raysearch Laboratories) for the development of a dedicated liver ablation planning and assessment software. “We are using this software to simulate and evaluate intraprocedurally the treatment endpoint, because we know that when we perform a liver ablation, the most important thing that we can do to improve local tumour control is to effectively cover the tumour with adequate margins in all planes,” he explains.
Odisio and colleagues are enrolling for a phase II trial (COVERALL) to study how well software-aided imaging works in confirming tumour coverage with ablation on patients with liver tumours. The primary objective of the COVERALL trial is “To evaluate if the intraprocedure feedback of a biomechanical deformable registration volumetric image method during percutaneous ablation will increase the minimal ablation margins on a three-dimensional (3D) computed tomography (CT)-generated analysis.”
Clinicaltrials.gov registers: “The current standard for targeting tumour cells and evaluating the outcome of a liver ablation procedure is a visual inspection of the pre- and postprocedure computed tomography (CT) scans. Software-aided imaging systems (such as Morfeus, the one utilised in the COVER-ALL study) may help to improve the accuracy and effectiveness of liver ablation.”
Prior to receiving the NIH grant for the COVER-ALL study, Odisio and Brock conducted a preliminary, retrospective analysis using their software to make a fusion image of the preprocedural and postprocedural CT images. The software utilises a biomechanical model to align the images, which Odisio says allows them to account for patient breathing, positioning, and tissue desiccation associated with the ablation itself. “We noticed that many times, our simple visual inspection of the CT scans before and after the ablation gave us a false sense of confidence regarding covering the entire tumour. In fact, among the patients who had recurrence after the ablation, we noticed based on our software assessment that ablation did not cover the tumour properly in all three-dimensional planes. Also, usually the lack of sufficient ablation margins occurs in an oblique plan between the axials, coronal, and sagittal plans”. He adds: “Based on what we have learned so far, many of the cases we define as tumour recurrence are in fact residual unablated tumour. That was our motivation to use the software.” Using the software with biomechanical modelling negates the issues with trying to decipher simple axial imaging in a complex 3D environment.
He adds that it is very hard to analyse the imaging data intraprocedurally: “You have a constrained timeline; you have too much data for you to run. Our software uses artificial intelligence [AI] and a biomechanical model to analyse the data on probe placement and ablation margins during the liver ablation. We hope that this will solidify the idea that using software really helps to improve treatment endpoints.” This work is part of the Image Guided Cancer Therapy (IGCT) Research Program run through The University of Texas MD Anderson Cancer Center.
Luigi Solbiati and his team at Humanitas University in Milan, Italy, have also developed software to aid ablation (Ablation-fit, R.A.W. Srl). “If interventional radiologists wish to compete with surgeons, the availability of software enabling an accurate comparison of pre- and postinterventional CT or magnetic resonance imaging (MRI) scans must become mandatory and routinely employed,” Solbiati opines.
Describing the software to this newspaper, he comments: “It is based on automatic segmentation of liver parenchyma and major blood vessels, semi-automatic segmentation, and reconstruction of tumoural targets and post-ablation necrotic areas and [employs a] non-rigid, fully automatic registration of pre- and postablation CT scans that allows us to deform the liver parenchyma section by section, based on the location of intrahepatic blood vessels. This allows for an extremely precise image fusion regardless of differences in body position, respiratory motion, or liver deformation. In addition, in very short time (five to 10 minutes) the software calculates the percentages of residual, unablated volumes of both the target tumour and predetermined 3D safety margin.”
In a retrospective study of 90 hepatocellular carcinomas (HCCs) that had undergone microwave ablation with apparent technical success and followed for at least one year, published in the International Journal of Hyperthermia, Solbiati et al demonstrated that, if they could have used this software, they would have discovered incomplete treatments at 24-hour CT scan (enabling immediate re-treatment) in 76.6% of the HCCs that actually had local tumour progression at one year.
In a clinical study recently published in European Radiology of colorectal liver metastases that have undergone radiofrequency ablation (RFA) with volumetric assessment of the periablational safety margin performed using this software, it was proved that the safety margin assessment was the only independent predictor of local tumour progression, and that at least 90% of a 6mm circumscribed 3D safety margin was required to achieve complete ablation.
“Ablation-confirmation software like this can be used in different modalities,” Solbiati says. “When ablations are performed in sonographic rooms under the control of contrast-enhanced ultrasound (US), potentially integrated with real-time US-CT/MRI image fusion, ablation-confirmation software like this can be used only after the end of the treatment. In this situation, a level of assessment closer to that achieved by pathologists after surgical resection can be obtained, but if incomplete treatment or insufficient ablative margins are seen, the procedure will have to be repeated, as it would occur for surgery. If ablations are performed under US guidance (or CT guidance) in the CT room (as would be recommended nowadays), the software would be used immediately, during the procedure, and the treatment could be immediately completed if partial or with insufficient ablative margins. This would represent even an advantage over surgical resection.
“In addition, this kind of software can trace out the ideal path to the portion of tumour to be treated, thus significantly facilitating the completeness of the procedure.
“In the near future, it is hoped that such easily usable and extremely fast software will be increasingly employed in interventional departments, while we are currently working to extend their applicability also to extrahepatic organs (kidney, lung, lymph nodes, prostate) and to MRI scans.”
ACCLAIM and COLLISION: Trial to watch
The SIO is also currently planning a multicentre clinical trial—the ACCLAIM trial—that incorporates software that looks at margins into the ablation, and which will aim to determine if achieving complete margins during an ablation equates to procedural success, assessed through disease progression or recurrence postprocedurally. Constantinos Sofocleous (Memorial Sloan Kettering Cancer Center, New York, USA) will be the principal investigator.
“The ACCLAIM trial will be the first liver ablation trial to use a well-defined objective technical outcome measure as a critical aspect of the trial design,” Callstrom tells Interventional News. “We anticipate that this will help drive adoption of this approach as the standard for liver mass ablation”.
Sofocleous comments: “The ACCLAIM trial is a single-arm, prospective, multicentre, international phase II study where thermal ablation will be offered in patients with limited number and relatively small CRC [colorectal cancer] liver metastases. A mandatory requirement for enrolment in the trial is the use of real-time 3D software to assess the ablation zone and show that the target tumour has been completely covered with ideally 10mm and not less than 5mm of minimal ablation margins. The study hypothesis is that if ablation margins are larger than 5mm, local progression-free survival at 12 months after ablation will be over 85%.”
Another hotly anticipated trial is the COLLISION trial. Sofocleous tells this newspaper: “I am very interested to see the results of the COLLISION trial that compares thermal ablation to resection for well-selected small colorectal liver tumours. This trial is now enrolling in The Netherlands, and will address a lot of the questions that exist for the value of ablation. I am particularly interested to find out whether the trial will provide high-level evidence about thermal ablation and whether this treatment can be equally effective and potentially safer than limited resection as a local curative therapy for CRC liver metastases.”
In an additional attempt to further improve margin confirmation, Solomon informs this newspaper that he and Sofocleous are conducting research into performing biopsies around the edges of the tumour intraoperatively, in order to gain a quick pathological assessment of whether or not any cancer cells are left alive. This allows them to be more adaptive during the procedure: “If, during the procedure, we find there is still live tissue, we would do something about it. If the software shows us that the margins are close, maybe we would expand it [the ablation zone] a bit. All of these things allow us to finesse and improve our results.”












Liver changes its shape during ablation and it is really hard to believe that any software can follow that deformation with 1-2 mm precision (which is what we need in ablations).