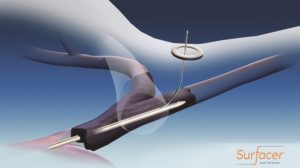
Bluegrass Vascular Technologies has announced that the first US commercial cases using the Surfacer Inside-Out access catheter system were completed at Santa Clara Valley Medical Center in San Jose, California.
The procedures were performed by Ehab Sorial and Ajit Nair. Sorial is a clinical associate professor of Surgery in the Division of Vascular Surgery at Stanford University School of Medicine and staff surgeon at Santa Clara Valley Medical Center. Nair is a staff interventional radiologist at Santa Clara Valley Medical Center.
The device was used to obtain venous access in two haemodialysis patients with central venous obstructions, one of which had limited access options. Bluegrass Vascular was granted de novo classification by the US Food and Drug Administration (FDA) for the Surfacer system in February of this year.
“We are excited to be the first programme in the USA to incorporate use of the Surfacer system into our day-to-day clinical practice,” said Nair and Sorial. “The Surfacer system provides us with a safe and effective option for obtaining right-sided central venous access in patients with venous obstructions, enabling us to preserve secondary central veins and avoid alternative access procedures, which are not as effective and have a higher risk of complications.”
“Dr Sorial, Dr Nair and their team at Santa Clara Valley Medical Center played an integral role as one of several clinical sites for the US investigational device exemption (IDE) study, which led to our recent FDA de novo clearance. We are honoured to have Drs Sorial and Nair perform our first US cases,” stated Gabriele Niederauer, CEO and president of Bluegrass Vascular. “We envision US clinical adoption of the Surfacer system mirroring our positive commercial experiences in Europe and look forward to providing this truly novel technology to physicians and their patients across America.”











