
Six-month results of the WRAPSODY Arteriovenous Access Efficacy (WAVE) pivotal trial have reported superior target lesion primary patency (TLPP) with Wrapsody (Merit Medical) cell-impermeable endoprosthesis (CIE) when compared to percutaneous transluminal angioplasty (PTA). These data were presented by Mahmood Razavi (St Joseph Heart & Vascular Center, Orange, USA) during a FIRST@CIRSE session at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress (14–18 September, Lisbon, Portugal).
Wrapsody was developed to aid physicians in treating patients with stenosis/occlusion in the vessels used for haemodialysis. Creation and maintenance of an arteriovenous fistula or graft (AVF/AVG) to achieve long term vascular access is required for patients undergoing haemodialysis, although progressive stenosis or occlusion can pose life-threatening consequences.
In 2021, clinical outcomes from the first-in-human trial of Wrapsody showed a 12-month TLPP rate of 84.6% and an access circuit primary patency (ACPP) of 65.9%. Building on these results, the WAVE trial was conducted to assess the outcome of Wrapsody endoprosthesis as compared to PTA in a large-scale study.
The prospective, randomised, multicentre study enrolled 245 patients with arteriovenous fistula (AVF) peripheral venous circuit outflow lesions requiring intervention from 43 international centres. Of this cohort, 122 patients were randomised to PTA and 123 to Wrapsody.
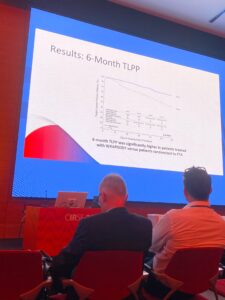 Their results showed that six-month patency was “significantly” higher for both TLPP (89.8% vs. 62.8%, p<0.0001) and ACPP (72.6% vs. 57.9%, p<0.015) in patients treated with Wrapsody when compared with patients randomised to PTA. Additionally, in a summary of safety events through 30 days post treatment, there was no significant difference between the groups with very low event rates in both. Freedom from 30-day safety event rate was 96.6% in the Wrapsody group versus 95% in the PTA group.
Their results showed that six-month patency was “significantly” higher for both TLPP (89.8% vs. 62.8%, p<0.0001) and ACPP (72.6% vs. 57.9%, p<0.015) in patients treated with Wrapsody when compared with patients randomised to PTA. Additionally, in a summary of safety events through 30 days post treatment, there was no significant difference between the groups with very low event rates in both. Freedom from 30-day safety event rate was 96.6% in the Wrapsody group versus 95% in the PTA group.
Speaking to Interventional News following his presentation, Razavi noted that Wrapsody’s superiority over PTA concerning ACPP is crucial, as this is a “critical” metric for physicians and patients when identifying the magnitude of an intervention’s benefit.
He continued, stating that: “While covered stents and drug-coated balloons have improved lesion patency in these patients, ACPP still remains a challenge. Results of this trial not only showed superiority of Wrapsody over angioplasty but also numerical improvement over prior technologies. This six-month analysis provides insight regarding Wrapsody’s anticipated long-term performance.”










