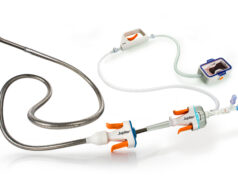
The US Food and Drug Administration has given 510(k) clearance to Galil Medical to market its Visual-ICE Cryoablation System.
The new system, recently showcased at SIR 2012, promote more precision and flexibility for the user, allowing for increased control over the shape and size of individual iceballs. Further, the system simplifies and shortens cryoablation procedures, including the operation and monitoring of the procedure from a large, single touch-screen.
The Visual-ICE Cryoablation System is the first oncology focused cryoablation system introduced in over six years, engineered with multiple physician specialties in mind, and will be the platform technology for the company’s next generation needle products.
Faster overall procedure times, the ability to concurrently treat larger and/or multiple lesions are some of the additional features of the Visual-ICE system. The new system will also leverage i-Thaw, the company’s proprietary argon-only needle technology, resulting in lower operating costs for hospitals.
“The Visual-ICE System represents the latest example of our commitment to advancing cryotherapy,” commented Martin J Emerson, president and CEO, Galil Medical. “Our goal with the Visual-ICE System is to enhance the user experience. Through state of the art software and technology, this new system will provide the user with more intra-procedural precision and control. We look forward to meeting the needs of our physician customers for many years to come.”
Galil Medical will showcase their new system at the upcoming European Congress of Interventional Oncology meeting, the American Urological Association meeting and the 5th International Symposium on Focal Therapy and Imaging in Prostate and Kidney Cancer.