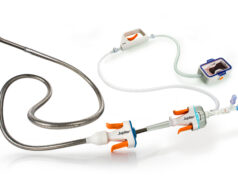
Vesalio recently announced two new US Food and Drug Administration (FDA) 510(k) clearances for its aspiration devices, designed for peripheral and neurovascular applications.
Vesalio recently announced two new US Food and Drug Administration (FDA) 510(k) clearances for its aspiration devices, designed for peripheral and neurovascular applications.
By integrating aspiration technology with its flagship mechanical retrieval platform, powered by proprietary Drop Zone and microfiltration technologies, Vesalio states that it delivers a versatile armamentarium for efficient clot removal. The company notes that this expanded portfolio builds on proven stent-based innovations such as NeVa, enVast, and pVasc, which have demonstrated success across neuro, coronary, and peripheral anatomies and are already adopted in global markets.
“With these new aspiration clearances, we’re uniquely positioned to provide physicians broader options to address diverse clot morphologies, driving better patient outcomes in critical conditions like stroke, myocardial infarct, and acute limb ischaemia,” said Steve Rybka, CEO of Vesalio. “We will continue to broaden our portfolio with advanced solutions, applying our expertise to transform outcomes in additional disease states to make a meaningful impact on patient lives.”