With transarterial radioembolization, one area that continues to be shrouded in debate is the concept of dosimetry and dose, writes David Liu.

As we develop a deeper understanding of the power and potential of transarterial radioembolization (TARE), we have continued to refine both the strategy of patient selection, and improve upon the tactical execution of its delivery. Clearly, with the refined protocols and dedicated devices that are available, we are doing better. However, the one area that continues to be shrouded in debate is the concept of dosimetry and dose.
Although the term ‘dose’ within the radioembolization vernacular refers to the administration of a vial or vials of radioactive microparticles, and this is incorrect.
We can only address the issues of dose by understanding some basic physical principles.
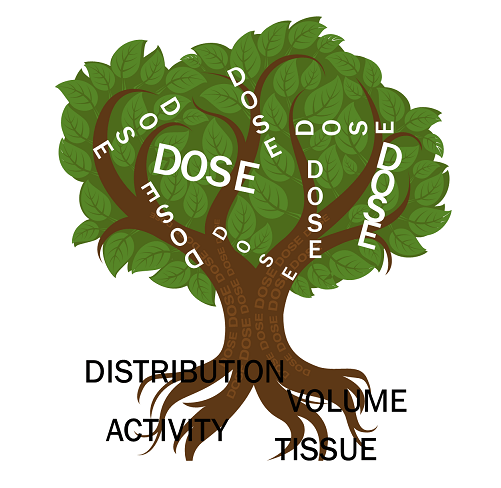
The particle itself is essentially a benign carrier. When performing TARE, activity (measured in decays per seconds, or Becquerel [Bq]) is administered intra-arterially, resulting in deposition into the terminal vascular bed of the said vessel. Not all of the spheres arrive at the tumour, and as a result, additional collateral damage to the liver parenchyma, lungs, and in some cases other non-targeted tissue (such as stomach) occurs, resulting in these unintended compartments receiving different amounts and distribution of activity. Dose is dependent on four fundamental factors: activity, volume, distribution and susceptibility of the exposed tissue and can be derived for the tumour, liver parenchyma and other volumes of tissue (eg lung). When these four basic factors are taken into account, we may derive the dose (measured in absorbed radiation in tissue, with Gray (Gy) as the most commonly used unit of measurement) and this practice is termed dosimetry. When introducing these concepts to those that are new to dose, I describe these factors as fundamental roots in the creation of a ‘dosimi-tree’ (Figure 1).
The aforementioned physical principles are not incorporated into the commonly used methods of determining the amount of radioactivity to inject. The Medical Imaging Radiation Dose (or MIRD), and Body Surface Area (BSA) methods do not accurately account for distribution, and susceptibility of tissue to radiation. Thus, controversial issues distill into arguments of conjecture (eg too many particles/too few particles, how to adjust for damaged liver, and more/less activity being more/less effective). When one examines the physical differences between commercially available glass (ie More radiation per particle, more overall radiation injected during a procedure, and less particles overall) and commercially available resin (ie Less radiation per particle, less overall radiation injected during a procedure, and more particles overall), the concept of dose becomes more complex. This situation is clearly demonstrated when more advanced methods of dose calculation (using the Partition method) result in the high variation of what is considered a tumouricidal dose. Wide ranges of optimal tumour dose, from 100–120Gy for resin, to 200–500Gy for glass are reported, all generally resulting in very similar response and survival (at least in hepatocellular carcinoma).
In summary, the activity is fundamentally bound to the particle, so the particle dictates the distribution, and only when this is married with the target tissue susceptibility, and target volume, may dose be derived. In this next level of discussion, apps such as the iOS Dosimetry & Activity Visualizer for Y90 Radioembolization [DAVYR] (available for free download on the Apple App Store for iOS Devices, search term: DAVYR) will be essential to developing personalised treatment plans. As we continue to mature TARE, progress to a common lexicon and common goals must be recognised: we must minimise non-targeted radiation (protect the liver, and protect the lungs), minimise overall activity to be injected (As Low As Reasonably Acceptable [ALARA]), and optimise (not maximise) dose to tumour.
David Liu is a clinical associate professor, Faculty of Medicine, University of British Columbia, Vancouver, Canada. He has acted as a consultant for Sirtex Medical and BTG.