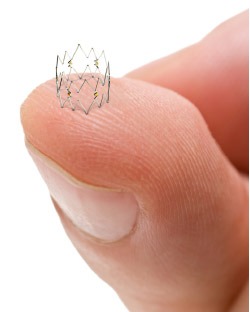
The Tack Optimized Balloon Angioplasty II (TOBA II) clinical trial has successfully achieved both primary and secondary endpoints. One year results from the TOBA II study were presented in the late-breaking scientific session by William Gray, system chief, Division of Cardiovascular Disease at Main Line Health, president, Lankenau Heart Institute and principal investigator for the TOBA II trial at VIVA (5–8 November, Las Vegas).
The first peripheral vascular study to enrol patients with 100% dissected vessels, the TOBA II study, was conducted at 33 US and European sites to investigate the Tack Endovascular System (Intact Vascular) for the repair of post-angioplasty dissections in femoropopliteal arteries. All patients enrolled (n=213) suffered from peripheral arterial disease (PAD), underwent balloon angioplasty with either plain or drug-coated balloons, and consequently experienced at least one dissection, with 69.4% being classified as severe.
Results presented from this first-of-its-kind study demonstrated 92.1% complete dissection resolution within a clinically challenging patient population, along with 79.3% K-M vessel patency and 86.5% K-M freedom from clinically driven reintervention at 12-months. Additionally, the TOBA II study validated the Tack implants as stable and durable, with zero implant fractures, 99.9% freedom from migration, and a 0.5% bailout stent rate.
“The TOBA II study is unique in that it is the first large scale pivotal evaluation in peripheral arterial vessels that are 100% dissected following initial angioplasty and treated with a precisely targeted implant,” commented William Gray. “This study introduces a new therapeutic paradigm, demonstrating that we can repair dissected arteries, leaving minimal metal behind to preserve future treatment options for our patients, and producing excellent 12-month outcomes.”
“As presented today, the TOBA II data further augment the growing body of evidence supporting the idea that a purpose-built implant for dissection repair can enhance the clinical results of balloon angioplasty,” said Bruce Shook, Intact Vascular’s president and CEO. “The Tack Endovascular System is also well aligned with a primary goal of endovascular operators: to leave as little metal behind as possible.”












