Tag: VTE
One year on: Matthew Johnson reflects on the PRESERVE trial
“Is more research needed in the world of filters? Yes, but is more research needed to determine whether the class itself is safe? No.”...
Endovascular Engineering announce first patient enrolled in PE thrombectomy trial
Endovascular Engineering, a medical device company involved with clot removal technologies for venous thromboembolism (VTE), has announced the enrolment and treatment of the first...
Inari Medical announces commercial launch of RevCore and Triever16 Curve for...
Inari Medical today announced the launch of two new purpose-built products, the RevCore thrombectomy catheter, and the Triever16 Curve catheter.
According to a company press...
Akura Medical announces successful first-in-human use of its mechanical thrombectomy platform
Akura Medical announced today it has initiated its first-in-human clinical study of the Akura mechanical thrombectomy platform. A press release notes that the Akura...
New study demonstrates IVC filters “safe and effective” in treating venous...
Few adverse events are connected to the use of inferior vena cava (IVC) filters to help prevent deep vein blood clots from developing into...
Cordis makes strategic investment in E2, a developer of next-generation thrombectomy...
Cordis has announced a strategic investment venture that will expand the scope of the global cardiovascular technology company into the venous thromboembolism (VTE) market...
Prolonged TV watching may increase risk of venous thromboembolism
A new study reports that watching TV for four hours a day or more is associated with a 35% higher risk of venous thromboembolism...
New study addresses thromboembolic complications in COVID-19 patients
There is an “urgent need” to improve specific venous thromboembolism (VTE) diagnostic strategies and investigate the efficacy and safety of thromboprophylaxis in ambulatory COVID-19...
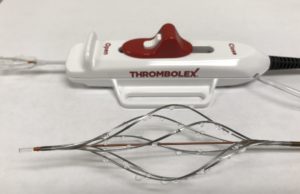
FDA approval granted to Thrombolex for Bashir Endovascular Catheter-Short Basket
Thrombolex has announced that the company has received US Food and Drug Administration (FDA) clearance for the Bashir Endovascular Catheter - Short Basket (BEC...
FDA approves new 10mg dosing for rivaroxaban to reduce continued risk...
Janssen Pharmaceuticals has announced that the FDA has approved the 10mg once-daily dose of rivaroxaban (Xarelto) for reducing the continued risk for recurrent venous...