Tag: pulmonary embolism
CAVT with anticoagulation significantly improves functional outcomes in PE patients, RCT...
Mechanical thrombectomy by means of computer-assisted vacuum thrombectomy (CAVT) using the 16Fr Lightning Flash system (Penumbra) along with anticoagulation for acute-to-intermediate pulmonary embolism (PE)...
PE thrombectomy: ENGULF pivotal study meets safety and efficacy endpoints
Full results from the ENGULF pivotal study of the Hēlo thrombectomy system (Endovascular Engineering) in acute intermediate risk pulmonary embolism (PE) patients demonstrated a...
Interim RAPID-PE study reveals ‘excellent’ safety data in first 50 patients
Prespecified interim analysis data from the first 50 patients in RAPID-PE have demonstrated "excellent safety" and "remarkably efficient lab times" in patients receiving on-the-table...
STORM-PE finds mechanical thrombectomy superior to anticoagulation alone
The use of mechanical thrombectomy, specifically computer-assisted vacuum thrombectomy (CAVT) using the 16Fr Lightning Flash system (Penumbra), with anticoagulation achieves superior reduction in right...
Imperative Care receives FDA 150(k) clearance for Symphony thrombectomy system
Imperative Care has today announced US Food and Drug Administration (FDA) 510(k) clearance of its Symphony thrombectomy system to treat pulmonary embolism (PE).
This clearance...
First patient enrolled in RECOVER-AV trial of AlphaVac F18 system for...
AngioDynamics has announced the first patient has been enrolled in the RECOVER-AV clinical trial, a prospective, multicentre, international, single-arm study evaluating the AlphaVac multipurpose...
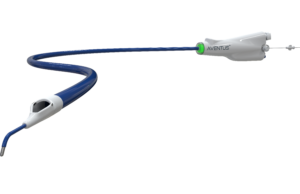
Aventus thrombectomy system gains FDA clearance for PE treatment
Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded...
Penumbra’s STORM-PE randomised trial completes enrolment
Penumbra has announced the completion of enrolment in the STORM-PE clinical trial.
The pivotal, prospective, multicentre randomised controlled trial enrolled 100 patients to evaluate computer...
STRIKE-PE interim analysis shows benefit for acute PE patients with syncope
A STRIKE-PE interim analysis of clinical outcomes of acute pulmonary embolism (PE) patients with or without syncope—an indicator of compromised cardiac output and so...
Akura Medical enrols first patient in QUADRA-PE study
Akura Medical has announced today the first patient enrolment in the QUADRA-PE study evaluating the Katana thrombectomy system in patients with acute pulmonary embolism...
APEX-AV trial results of AlphaVac PE system published in JSCAI
AngioDynamics has announced the publication of the results from the Acute Pulmonary Embolism Extraction Trial with the AlphaVac system—APEX-AV—in the Journal of the Society...
AVENTUS trial of thrombectomy system for PE treatment completes enrolment
Inquis Medical has announced completion of patient enrolment in its AVENTUS clinical trial, a pivotal investigational device exemption (IDE) trial evaluating the safety and...
Argon Medical enrols first patient in CLEAN-PE study
Argon Medical announces the first patient enrolment in the CLEAN-PE study. The prospective, multicentre CLEAN-PE study aims to evaluate the safety and efficacy of...
PEERLESS trial finds large-bore mechanical thrombectomy superior for intermediate-risk PE
Findings from the first international randomised controlled trial (RCT) to compare patient outcomes following treatment with large-bore mechanical thrombectomy (LBMT) versus catheter-directed thrombolysis (CDT)...
First US cases performed with Vertex PE system in SPIRARE II...
Jupiter Endovascular has announced that the first US patient has been treated in the SPIRARE II US pivotal study of the Vertex pulmonary embolectomy...
First cases completed in SPIRARE I trial with Vertex system
Jupiter Endovascular has announced that the first two patients have been treated in SPIRARE I, a multicentre study of the Vertex pulmonary embolectomy system...
Ensuring the right treatment for patients with PE and DVT
In this short video, Michael Kostrzewa (Baden, Switzerland) and Rashid Akhtar (London, UK) discuss the latest approaches to the treatment of acute pulmonary embolism...
Mechanical thrombectomy shifts the needle toward interventional management of PE and...
This advertorial is sponsored by Inari Medical.
Evaluating the current standard of care for the management of acute pulmonary embolism (PE) and deep vein thrombosis...
Inari Medical updates ClotTriever XL IFU amid US FDA recall notice
The US Food and Drug Administration (FDA) has issued a Class I recall—the most serious type—for Inari Medical's ClotTriever XL, 30mm device due to...
Thrombectomy gold rush set to follow ATTRACT study, experts argue
At the recent Endo Vascular Access (EVA) meeting (14–15 June, Patras, Greece), Ziv Haskal (University of Virginia School of Medicine, Charlottesville, USA) spoke to...
AngioDynamics announces CE mark approval for AlphaVac system
AngioDynamics, a leading medical technology company focused on restoring healthy blood flow in the body’s vascular system, has announced European CE mark approval of...
Women and black patients less likely to receive catheter-based treatment for...
New data from the REAL-PE analysis investigated catheter-based pulmonary embolism (PE) treatment, showing women and Black people were less frequently treated with minimally invasive...
Penumbra announces FDA clearance of Lightning Flash 2 for the treatment...
Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of Lightning Flash 2, the next generation computer assisted vacuum thrombectomy (CAVT)...
AngioDynamics receives 510(k) clearance for AlphaVac system in treatment of PE
AngioDynamics, a leading and transformative medical technology company focused on restoring healthy blood flow in the body’s vascular system, expanding cancer treatment options and...
Avicenna.AI receives FDA 510k approval for PE and stroke severity technology
Medical imaging artificial intelligence (AI) company Avicenna.AI have today announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA)...
One year on: Matthew Johnson reflects on the PRESERVE trial
“Is more research needed in the world of filters? Yes, but is more research needed to determine whether the class itself is safe? No.”...
Endovascular Engineering announce first patient enrolled in PE thrombectomy trial
Endovascular Engineering, a medical device company involved with clot removal technologies for venous thromboembolism (VTE), has announced the enrolment and treatment of the first...
First patient enrolled in STORM-PE RCT evaluating Penumbra’s Lightning Flash for...
Penumbra today announced that the first patient has been enrolled in STORM-PE, a prospective, multicentre, randomised controlled trial (RCT) evaluating anticoagulation alone versus anticoagulation...
Computer-assisted vacuum thrombectomy (CAVT) system ‘revolutionises’ pulmonary embolism treatment
This advertorial, sponsored by Penumbra, is intended only for readers outside the USA.
“The advances led by this technology have significantly revolutionised our procedures,” states...
Interventional News’ top 10 most popular stories of October 2023
Interventional News’ most read stories in October included significant data releases from TCT 2023 (23–26 October, San Francisco, USA); results from a study investigating sex...
Interventional News Issue 92—November 2023
Interventional News 92 Highlights:
International IR voices map the path to primary specialty
Interventional News welcomes new editor-in-chief Robert Morgan
Profile: Jonathan Moss
CIRSE: Top...
Late breaking STRIKE-PE data shows improved outcomes for pulmonary embolism patients
Penumbra, a global healthcare company focused on innovative therapies, announced the latest STRIKE-PE data evaluating Penumbra’s Indigo aspiration system with Lightning. The results show...
E2 shares positive initial results from use of Hēlo system for...
Endovascular Engineering (E2) has announced positive initial results from its ENGULF study, demonstrating successful outcomes among the first 15 patients evaluated in the trial...
Study provides insights from US cohort of FLASH registry for high-risk...
New research from the FLASH registry shines a light on the effectiveness of large-bore mechanical thrombectomy in managing high-risk pulmonary embolism. The study, titled "Mechanical thrombectomy for high-risk pulmonary embolism:...
REAL-PE demonstrates statistically significant lower major bleeding rates with Ekos system...
Data from the REAL-PE study were presented this week at TCT 2023 (23–26 October, San Francisco, USA) demonstrating that patients treated for pulmonary embolism...
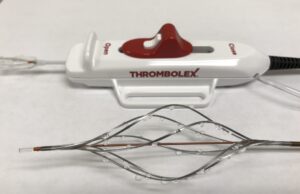
Thrombolex announces new insights from the RESCUE trial with the Bashir...
Thrombolex has announced never-before-reported major reductions in obstruction in all of the segmental pulmonary arteries (PA), based on independent core lab data analysis of...
“Rapid improvement” in right ventricular metrics following computer-aided mechanical aspiration thrombectomy
Presenting real-world population data in patients with pulmonary embolism (PE), John Moriarty (University of California, Los Angeles Medical Center, Los Angeles, USA) demonstrated results...
Inari Medical announces commercial launch of RevCore and Triever16 Curve for...
Inari Medical today announced the launch of two new purpose-built products, the RevCore thrombectomy catheter, and the Triever16 Curve catheter.
According to a company press...
Akura Medical announces successful first-in-human use of its mechanical thrombectomy platform
Akura Medical announced today it has initiated its first-in-human clinical study of the Akura mechanical thrombectomy platform. A press release notes that the Akura...
Viz.ai to expedite patient enrolment in NIH-funded PE-TRACT clinical trial
Viz.ai has announced it will use its Viz Recruit platform to optimise patient enrolment for the National Institutes of Health (NIH)-funded Pulmonary embolism—thrombus removal...
New study demonstrates IVC filters “safe and effective” in treating venous...
Few adverse events are connected to the use of inferior vena cava (IVC) filters to help prevent deep vein blood clots from developing into...
Cardiovascular Systems announces US FDA 510(k) submission of Innova Vascular’s thrombectomy...
Cardiovascular Systems (CSI) announced that Innova Vascular (Innova) has submitted a 510(k) premarket notification to the US Food and Drug Administration (FDA) for its...
FLASH results demonstrate “excellent safety profile” of the FlowTriever system in...
Results of the FLASH registry demonstrate the “excellent safety profile” of the FlowTriever system (Inari Medical) in 800 “real-world” patients. This is according to...
Penumbra and Asahi Intecc partner to introduce Indigo System to Japan
Penumbra and Asahi Intecc, a Japanese medical device manufacturer, announced that they will collaborate to introduce Penumbra’s Indigo Aspiration System into the Japanese market...
Database study could open up field for catheter-directed PE treatment research
A database study titled ‘Unplanned 30-day readmissions and in-hospital outcomes for the management of submassive and massive acute pulmonary embolism: Catheter-directed versus systemic thrombolysis’...
Magneto Thrombectomy Solutions announces successful first-in-human results for treatment of pulmonary...
Magneto Thrombectomy Solutions (Magneto), a medical devices company developing thrombectomy solutions for the treatment of ischaemic stroke and pulmonary embolism, presented successful first-in-human (FIH)...
Penumbra announces the European launch of the Indigo system with Lightning...
Penumbra has announced that its Indigo aspiration system with Lightning 7 and Lightning 12 have secured CE mark and are now commercially available in...
Study finds increased risk of serious blood clots up to six...
A study from Sweden published by The BMJ recently finds an increased risk of deep vein thrombosis (DVT) up to three months after COVID-19 infection,...
Cordis makes strategic investment in E2, a developer of next-generation thrombectomy...
Cordis has announced a strategic investment venture that will expand the scope of the global cardiovascular technology company into the venous thromboembolism (VTE) market...
Aidoc extends scope of AI solutions for medical imaging into the...
Aidoc, a technology company that provides artificial intelligence (AI) solutions for medical imaging, including in the cardiovascular space, is extending its services beyond the...
First patient enrolled in PEERLESS study of FlowTriever system
Inari Medical has announced that the first patient has been enrolled in PEERLESS prospective, randomised controlled trial (RCT) comparing the outcomes of patients with...
Interventional News’ top 10 most popular items of January 2022
January’s top 10 features technological advancements, with Cook Medical receiving US Food and Drug Administration (FDA) approval for new drug-eluting stents and Adept Medical...
Interventional News’ top 10 most popular items of January 2022
January’s top 10 features technological advancements, with Cook Medical receiving US Food and Drug Administration (FDA) approval for new drug-eluting stents and Adept Medical...
Thrombolex announces “very exciting” interim RESCUE results
Thrombolex recently announced the results of the RESCUE trial’s prespecified interim analysis, of the first 62 evaluable patients, in a late-breaking clinical trials session...
Viz.ai launches two new AI-powered modules for pulmonary embolism and aortic...
Viz.ai has announced the US commercial launch of its AI-powered modules for pulmonary embolism and aortic disease. Debuting at VEITHsymposium 2021 (16–20 November, Orlando, USA), the...
Inari Medical announces six-month FLASH registry interim data
Inari Medical has announced positive acute and long-term interim results from the first 500 pulmonary embolism (PE) patients enrolled in the FlowTriever outcomes registry...
Positive data for the EkoSonic endovascular system presented at VIVA 2021
Boston Scientific announced positive results for the EkoSonic endovascular system (EKOS system) during a late-breaking clinical trial presentation at Vascular Interventional Advances (VIVA) 2021...
Boston Scientific initiates randomised controlled trial for the EkoSonic endovascular system
Boston Scientific has commenced enrolment in the HI-PEITHO clinical trial, a collaborative research study with the Pulmonary Embolism Response Team (PERT) Consortium and the...
Meta-analysis: IVC filters should be considered for certain patients at high...
In a recent meta-analysis, Yang Liu, Huan Lu (Henan Cancer Hospital, Zhengzhou, China), and colleagues found insufficient evidence to prove that inferior vena cava...
EXTRACT-PE trial data confirm Indigo Aspiration system is safe and effective...
Data from the EXRACT-PE trial, published online first in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions, found that the Indigo...
Penumbra’s newest generation of Indigo aspiration system receives FDA clearance for...
Penumbra today announced US Food and Drug Administration (FDA) 510(k) clearance for expanded indication of the latest iteration of the Indigo aspiration system, Lightning...
Similar outcomes for ultrasound-assisted and standard thrombolysis in SUNSET sPE
Efthymios Avgerinos (University of Pittsburgh, Pittsburgh, USA) presented results from the SUNSET sPE trial during a late-breaking data session at this year’s Vascular Interventional...
SUNSET sPE thrombolysis trial completes enrolment phase
The “Standard versus ultrasound-assisted catheter thrombolysis for submassive pulmonary embolism” (SUNSET sPE) trial, a randomised, single-blinded clinical trial comparing ultrasound-assisted thrombolysis (USAT) to standard...
EXTRACT-PE an “important first study” for aspiration thrombectomy in acute PE...
Akhilesh Sista (New York, USA) talks to Interventional News at VIVA 2019 (Vascular InterVentional Advances; 4–7 November, Las Vegas, USA) about the results of the EXTRACT-PE study, that were presented in a late-breaking...
VIVA 2019: Penumbra Indigo Aspiration system IDE trial for acute PE...
Penumbra today announced that the EXTRACT-PE trial successfully met the primary endpoints, demonstrating the safety and efficacy of the Indigo Aspiration system for aspiration...
Thrombolex announces first enrolment in early feasibility and safety study using...
Thrombolex has announced the enrolment of the first patient in their Early Feasibility and Safety Study, investigating the Bashir Endovascular Catheter for the treatment...
Thrombus dissolution with EKOS™ Acoustic Pulse Thrombolysis™ in everyday clinical practice
At the EKOS symposium at LINC 2019, Nils Kucher (Zurich, Switzerland), Houman Jalaie (Aachen, Germany) and Mert Dumantepe (Istanbul, Turkey) share their experiences in...
BTG acquires Novate Medical
BTG has announced it has acquired Novate Medical, a medical device company focused on the prevention of pulmonary embolism in patients at high risk...
One-year OPTALYSE PE results reinforce safety and efficacy of shorter, lower...
The study results with the Ekos system (BTG) were presented at the International Society on Endovascular Therapy (ISET; 3–7 February 2018, Florida, USA).
The findings...
BTG receives US FDA 510(k) clearance for Ekos control unit 4.0
BTG has announced that US FDA 510(k) clearance has been granted to the Ekos control unit 4.0.
The Ekos system includes an ultrasonic device...
First US commercial placements of Angel catheter
Bio2 Medical has announced that the Angel catheter, a new device for the prevention of pulmonary embolism, was placed in two patients at St...