Tag: Fluidx Medical Technology
IDE pivotal trial for novel liquid embolic device completes enrolment
Fluidx Medical Technology has announced completion of patient enrolment in the investigational device exemption (IDE) pivotal clinical trial evaluating the GPX embolic device. The...
Fluidx Medical announces first patient treated in prospective trial
Fluidx Medical has announced that the first patient has been treated in the GPX investigational device exception (IDE) prospective multinational clinical trial.
The GPX...
Interventional News’ top 10 most popular items of February 2022
The February top 10 features news of the recent Japanese randomised control trial results, which support the use of mechanical thrombectomy in ischaemic stroke...
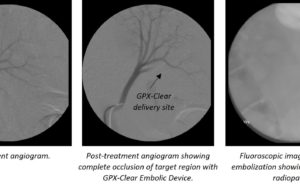
Fluidx Medical’s embolic device demonstrates promising visibility
Fluidx Medical Technology has announced the results of the GPX-Clear embolic device in-vivo research which uses the base GPX technology and incorporates an intermediate-term...
Fluidx Medical Technology announces Series A led by multinational strategic investor
Fluidx Medical Technology, has recently announced the oversubscribed closing of the first tranche of the Series A financing round. The Series A was led...
Novel embolic device used for arterial and venous tumour treatments
Fluidx Medical Technology has announced that GPX Embolic Device first patient use cases were presented at LINC 2021 (25–29 January, online).
“This is very promising...
First use of new embolic device for highly targeted tumour treatment...
A novel embolization device in the interventional oncology space—the GPX embolic device (Fluidx Medical Technology)—has successfully been used in a patient to therapeutically devascularise...