Tag: CE mark
Penumbra announces European launch of RED reperfusion catheters for stroke care
Penumbra announced today that its RED reperfusion catheters have secured a CE mark and are now commercially available in Europe. The catheters are part of...
Medtronic receives CE mark approval for radial artery access portfolio
Medtronic today announced it has received CE mark approval for its radial artery access portfolio, which includes the Rist radial access selective catheter and the Rist...
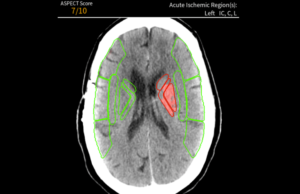
Avicenna.AI receives CE Mark for AI tool to assess stroke severity
Medical imaging AI specialist Avicenna.AI has received CE mark certification for its CINA ASPECTS AI tool for stroke severity assessment. CINA ASPECTS automatically processes...
Endologix receives CE mark for Alto abdominal stent graft system
Endologix recently announced that it has received a CE mark for the Alto abdominal stent graft system.
“We are very excited to receive a CE...
iVascular launches Sergeant peripheral support catheter
iVascular recently announced the launch of its Sergeant peripheral support catheter. Sergeant is a CE marked over-the-wire catheter, indicated for patients with peripheral arterial...
Merit Medical’s Wrapsody System receives CE mark
Merit Medical has received CE mark approval for the Wrapsody endovascular stent graft system from the British Standards Institution. The Wrapsody system is a...
XableCath crossing catheters have received CE mark for peripheral use
XableCath has announced that its XableCath Crossing catheters have received CE mark for peripheral use. Its crossing devices will be available for sale in...
Endologix announces reinstatement of CE mark for its Nellix endovascular aneurysm...
Endologix recently announced that the EC Certificate of Conformity (CE mark) for the Nellix endovascular aneurysm sealing system (Nellix system) has been reinstated by...
Guerbet receives CE mark approval for SeQure and DraKon microcatheters for...
SeQure and DraKon (Guerbet), two novel microcatheters for peripheral embolization procedures, are set for commercial launch in Europe. The devices have received the CE...
Nellix system CE Mark suspended
The CE Mark for the Nellix endovascular aneurysm sealing system (Endologix) has been suspended by its Notified Body, GMED, following a voluntary recall and...
First-ever CE mark of a bioresorbable scaffold for below-the-knee PAD
Reva Medical, a company developing bioresorbable polymer technologies for vascular applications, has announced that its Motiv bioresorbable scaffold is the first drug-eluting bioresorbable scaffold...
Terumo gets CE mark for Kanshas drug-coated balloon catheter for lower...
Terumo has announced receiving the CE mark for its Kanshas drug-coated balloon catheter used in the treatment of lower extremity peripheral arterial disease.
With...
Terumo Europe NV announces approval of LifePearl as a Class III...
This approval also expands the number of chemotherapeutic drugs that can be loaded onto LifePearl to include idarubicin and epirubicin, a press release from...
First European patients treated with DC Bead Lumi radiopaque drug-eluting beads
Two patients were treated for hepatocellular carcinoma and one patient was treated for malignant colorectal cancer metastasised to the liver.
DC Bead Lumi is...
Solero microwave ablation system gets CE mark
Angiodynamics has announced it has received CE mark certification for the Solero microwave tissue ablation system. Solero and its accessories are indicated for the...
Spectranetics gets CE mark for Stellarex 0.014” drug-coated balloon
Spectranetics has announced that its Stellarex 0.014” drug-coated angioplasty balloon has received the CE mark. The device is designed to treat small vessels, below-the...
Lumee Oxygen Platform for continuous, real-time monitoring of tissue oxygen receives...
Profusa has announced that receiving the CE mark to market its Lumee Oxygen Platform for continuous, real-time monitoring of tissue oxygen.
The company will initially...
Ra Medical receives approval in Europe for Dabra atherectomy system
Ra Medical has announced receiving CE mark approval for the Dabra atherectomy system with catheter.
Atherectomy is the removal of the plaque from the arteries,...