Tag: Catheter
Emboa Medical launches novel thrombectomy catheter for clot retrieval
Emboa Medical has announced a novel microstructured catheter which mimics the snake’s evolutionary advantage to improve the retrieval of blood clots that cause stroke.
The...
B Braun receives FDA clearance for deep access intravenous catheter
B Braun has announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the Introcan Safety 2 deep access intravenous...
Jupiter Endovascular announce US FDA approval for SPIRARE II trial
Jupiter Endovascular has announced that the US Food and Drug Administration (FDA) has approved its investigational device exemption (IDE) application for the SPIRARE II US pivotal study. The pivotal trial...
Penumbra announces European launch of neuro access catheters
Penumbra has announced that it has received CE mark approval and so has initiated the European launch of BMX81 and BMX96, devices which are...
Cook Medical announce partnership with catheter securement device company Bedal International
Cook Medical, a global medical device manufacturer, has partnered with Bedal International, a company specialising in catheter securement devices marketed under the brand FlexGRIP....
Merit Medical announces expanded microcatheter line for radial embolization
Merit Medical Systems, a leading global manufacturer and marketer of healthcare technology, today announced the expansion of its Maestro microcatheter product line to now...
Merit Medical announces series of dialysis catheter acquisitions
Merit Medical Systems has announced that it has completed the acquisition of a portfolio of dialysis catheter products and the BioSentry biopsy tract sealant...
Shape Memory Medical announces publication of results of embolization plug safety...
Shape Memory Medical has announced the publication of results from the company’s prospective, open-label, single-arm, safety study of the IMPEDE embolization plug in peripheral...
Interventional News’ top 10 most popular stories of March 2023
Interventional News’ most popular stories from March included those covering new embolization and radiofrequency ablation data presented at the Society of Interventional Radiology (SIR)...
RESCUE trial shows reduction in segmental and main pulmonary artery occlusions
Robert A Lookstein, who is executive vice chair, Diagnostic, Molecular and Interventional Radiology at the Icahn School of Medicine at Mount Sinai Hospital (New...
Database study could open up field for catheter-directed PE treatment research
A database study titled ‘Unplanned 30-day readmissions and in-hospital outcomes for the management of submassive and massive acute pulmonary embolism: Catheter-directed versus systemic thrombolysis’...
Northeast Scientific receives 510(k) for Turbo-Elite laser atherectomy catheter reprocessing
Northeast Scientific, a company that reprocesses single-use peripheral vascular catheters, announced recently in a press release that it has received US Food and Drug...
New retrospective data demonstrate midline catheters composed of advanced biomaterials may...
Access Vascular, Inc. (AVI) has shared initial findings from a retrospective review of patient records according to a press release. These demonstrate an increase...
Shimadzu Medical Systems announces release of new angiography system at SIR...
Shimadzu Medical Systems USA introduced the new Trinias system, which uses artificial intelligence deep learning to improve the visibility of medical devices while using lower...
Tractus Vascular announces first-in-man use of Tractus Crossing Support Catheter with...
Tractus Vascular recently announced the first-in-man use of the Tractus Crossing Support Catheter (Tractus CSC). The Tractus CSC represents a highly novel approach to...
Intermittent claudication: EffPac trial confirms benefit and safety of paclitaxel-coated balloon...
In the EffPac trial, designed to compare a drug-coated and an uncoated balloon in the treatment of vascular occlusion in the femoropopliteal region, the...
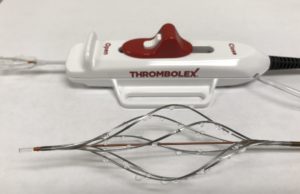
FDA approval granted to Thrombolex for Bashir Endovascular Catheter-Short Basket
Thrombolex has announced that the company has received US Food and Drug Administration (FDA) clearance for the Bashir Endovascular Catheter - Short Basket (BEC...
Merit Medical launches Resolve mini locking drainage catheter
Merit Medical has announced the commercial launch of the Resolve mini locking drainage catheter, the most recent addition to the company’s line of innovative...
Beacon Tip catheters available in Europe again
Beacon Tip catheters (Cook Medical) are once again available to physicians in Europe. As of September 2019, the catheters are available in the UK,...
New system for tracking catheters using optical fibres promises easier navigation
A new visualisation method enables more precise catheter navigation through the vascular system. It is being developed at the Fraunhofer Institute for Digital Medicine...
Proximo Medical selected as commercialisation partner for Walk Vascular
Proximo Medical has announced that it has been selected as the marketing and commercial partner for Walk Vascular, a medical device company that has...
NIH provides US$2.8 million grant to develop steerable robotic guidewire
The National Institutes of Health (NIH) National Heart, Lung and Blood Institute has awarded a US$2.8 million grant to support the development of an...
Biotronik expands peripheral portfolio with a new treatment tool for interventions...
Biotronik has launched the Carnelian support catheter, designed to improve the access for treatment of tortuous and highly calcified lesions. Carnelian support is indicated...
First patients enrolled in Wing-IT investigational device exemption clinical trial
Reflow Medical announced that the first patients have been enrolled in a prospective, multicentre, non-randomised study intended to evaluate the ability of the Reflow...
Hospital brings emergency REBOA procedure training to trauma US specialists
Grady Memorial Hospital, the first in Georgia, USA, to perform resuscitative endovascular balloon occlusion of the aorta (REBOA), will soon offer trauma specialists around...
New proprietary gel-coated biocompatible embolic, GPX, presented at LINC
Two scientific abstracts of a new vascular embolic device, GPX (Fluidx Medical Technology) were presented this week at the 2018 Leipzig Interventional Congress (LINC;...
XableCath gets US FDA clearance for broadly effective catheter aimed at...
XableCath has announced its XableCath blunt tip support catheter has received clearance from the US FDA. The blunt tip catheter facilitates true lumen passage...
Cook Medical announces Universa sets to streamline percutaneous drainage product line
Cook Medical has rounded out the Universa line with the introduction of two new sets for percutaneous urinary drainage.
Each set includes a catheter and...
Teleflex receives US FDA clearance for Arrow and TightTrack tunneler
Teleflex has announced that it has received US FDA 510(k) clearance to market its Arrow JACC with Chlorag+ard technology and TightTrack tunneler.
Arrow JACC...