Tag: BTK
SUCCESS PTA stratified analysis finds equal benefit for sirolimus DEB above...
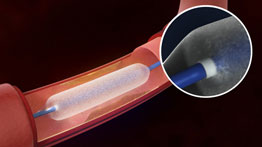
Positive 12-month outcomes for the Selution Sustained Limus Release (SLR; Cordis) drug-eluting balloon (DEB) were observed, with consistent haemodynamic, functional and clinical improvements irrespective...
US FDA grants R3 Vascular IDE approval for ELITE-BTK pivotal trial...
R3 Vascular today announced that the US Food and Drug Administration (FDA) has granted investigational device exemption (IDE) approval to initiate its ELITE-BTK pivotal...
Cordis announce positive 24-month results from Selution SFA Japan trial
Cordis has announced positive 24-month results from the Selution SFA Japan trial. The prospective, multicentre, single arm trial is designed to assess the safety...
Summa Therapeutics announce first-in-man injectable angioplasty treatments for PAD
Summa Therapeutics has announced that the first-in-man injectable angioplasty procedures for patients with below-knee peripheral arterial disease (PAD) were performed successfully using its Finesse...
Full cohort results confirms efficacy of Luminor drug-coated balloon for BTK...
iVascular reflect on the Luminor drug-coated balloon (DCB) in below-the-knee (BTK) outcomes obtained in the BIBLIOS trial one-year follow-up presented at the Paris Vascular...
“Excellent” 24-month MOTIV BTK outcomes for bioresorbable scaffold use in below-the-knee...
Presenting data from the MOTIV bioresorbable scaffold (Reva Medical) below-the-knee (BTK) trial during Saturday’s FIRST@CIRSE session at this year’s Cardiovascular and Interventional Radiological Society...
RECOIL study: Serranator demonstrates 49% less recoil than plain balloon angioplasty...
Cagent Vascular has announced the results of its below-the-knee (BTK) RECOIL study. This core lab-adjudicated Recoil analysis— the first of its kind, according to...
Concept Medical granted IDE approval for Magic Touch sirolimus DCB below...
The Magic Touch percutaneous transluminal angioplasty (PTA) sirolimus drug-coated balloon catheter (DCB) has received investigational device exemption (IDE) for the treatment of below-the-knee (BTK) atherosclerotic...
PRELUDE-BTK subanalysis “suggests advantage” for serration angioplasty
Cagent Vascular has announced the results of a comparative subanalysis of the PRELUDE-below-the-knee (BTK) study versus plain balloon angioplasty.
The study was led by Marianne...
Why laughter is not “frivolous”: Keeping burnout at bay in IR
Matthew Gibson (Royal Berkshire Hospital, Reading, UK) will be delivering this year’s British Society of Interventional Radiology (BSIR; 2–4 November, Glasgow, UK) Wattie Fletcher...
Six-month SWING trial data show promise for Sundance DCB
Six-month data from the Surmodics SWING first-in-human (FIH) study of the company’s Sundance sirolimus drug-coated balloon (DCB) were shared at the 2022 Amputation Prevention...
SAVAL trial finds no gains with custom drug-eluting stents in PAD...
Day one of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2022 annual meeting (10–14 September, Barcelona, Spain) put a strong focus on...
Selution SLR receives second FDA IDE approval
Selution SLR, MedAlliance's sirolimus-eluting balloon, has received conditional US Food and Drug Administration (FDA) investigational device exemption (IDE) approval to initiate its pivotal clinical...
Philips announces positive three-year clinical research results from its TOBA II...
Royal Philips today announced the latest results from the Tack optimised balloon angioplasty (TOBA) II below-the-knee (BTK) clinical trial, demonstrating that the Philips endovascular...
Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee...
R3 Vascular reports the initiation of its first-in-human clinical study
R3 Vascular has reported the successful initiation of its first-in-human clinical study evaluating the technical and clinical performance of the R3 Vascular Magnitude bioresorbable...
PRISTINE registry with Selution SLR sirolimus drug-eluting balloon completes enrolment
MedAlliance has announced completion of patient enrolment in the PRISTINE clinical trial with the Selution SLR 018 drug-eluting balloon (DEB) for the treatment of...
FDA advisory panel recommends against premarket approval of Lutonix 014 DCB...
A US Food and Drug Administration (FDA) advisory panel has recommended against premarket approval of BD’s Lutonix 014 drug-coated balloon (DCB) for use in...
LINC 2021: Novel approaches and new data for BTK interventions revealed
In a late-breaking trial session at LINC 2021 (The Leipzig Interventional Course, 25–29 January, online), key updates on below-the-knee (BTK) interventions were in the...
OUTBACK™ Elite Re-entry catheter highly effective at saving limbs in extreme...
Lorenzo Patrone (London, UK) shares with Blearning at CIRSE 2019 (7–11 September, Barcelona, Spain) the benefits of using the OUTBACK™ Elite Re-entry Catheter (Cordis,...
First-ever CE mark of a bioresorbable scaffold for below-the-knee PAD
Reva Medical, a company developing bioresorbable polymer technologies for vascular applications, has announced that its Motiv bioresorbable scaffold is the first drug-eluting bioresorbable scaffold...
BD announces completion of enrolment in Lutonix 014 DCB below-the-knee trial
Enrolment is complete in the Lutonix below-the-knee trial with BD planning to submit a pre-market approval application to the US Food and Drug Administration...
Positive 24-month TOBA results presented at VEITHsymposium
Intact Vascular has announced that positive single centre twenty-four month results from its TOBA (Tack optimised balloon angioplasty) clinical study were presented at VEITHsymposium...
Spectranetics gets CE mark for Stellarex 0.014” drug-coated balloon
Spectranetics has announced that its Stellarex 0.014” drug-coated angioplasty balloon has received the CE mark. The device is designed to treat small vessels, below-the...
Medtronic receives US FDA clearance for TrailBlazer angled peripheral support catheter
Medtronic has announced that the US FDA has cleared the TrailBlazer angled support catheter for use in the peripheral vascular system. Support catheters such...