Tag: BIOTRONIK
US FDA Breakthrough Device designation granted for Biotronik’s Freesolve BTK resorbable...
Biotronik has been granted Breakthrough Device designation (BDD) from the US Food and Drug Administration (FDA) for the Freesolve below-the-knee (BTK) resorbable magnesium scaffold...
Two-year BIOPACT randomised controlled trial analysis demonstrates “persistent excellence” for low-profile...
Biotronik has announced the presentation of two-year results from the investigator-initiated BIOPACT randomised controlled trial (RCT) by principal investigator Koen Deloose (AZ Sint-Blasius Hospital,...
Interventional News’ top 10 most popular stories of September 2023
Interventional News’ most read stories in September included first data releases from the this year’s Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2023 annual...
Biotronik announce two-year results from DCB catheter registry
Biotronik has announced the two-year results from the BIOLUX P-III BENELUX all-comers registry, presented by principal investigator Frank Vermassen (Ghent University, Gent, Belgium) at...
Interventional News’ top 10 most popular stories of July 2023
Interventional News’ most popular stories from July included point of view pieces from interventional radiologists on fallopian tube recanalisation and interventions for postpartum haemorrhage,...
Biotronik announces one-year subgroup results from BIOPACT RCT
Biotronik has announced one-year subgroup results from the investigator-initiated BIOPACT randomised controlled trial (RCT), which were presented by principal investigator Koen Deloose (AZ Sint-Blasius,...
Interventional News’ top 10 most popular stories of June 2023
Interventional News’ most popular stories from June included a letter from the joint editor-in-chief on the current state of interventional radiology (IR) practice and...
Biotronik launches Oscar multifunctional peripheral catheter at LINC 2023
Biotronik today announced the limited release of its Oscar (One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter and start of promotional activities at this...
BIOPACT RCT suggests Passeo-18 non-inferior to IN.PACT Admiral DCB
Koen Deloose (Dendermonde, Belgium) talks to Vascular News about the key one-year data from the BIOPACT randomised controlled non-inferiority trial, which evaluated the safety...
Interventional News’ top 10 most popular stories of February 2023
Interventional News’ most popular stories for the month of February included news from the Pan Arab Interventional Radiology Society annual meeting (PAIRS; 11–14 February,...
Biotronik launches Oscar multifunctional peripheral catheter
Biotronik has announced the US Food and Drug Administration (FDA) 510(k) clearance and CE mark of its Oscar (One Solution: Cross. Adjust. Restore) multifunctional...
Biotronik’s Pulsar-18 T3 peripheral self-expanding stent system receives FDA approval
Biotronik recently announced that it has received US Food and Drug Administration (FDA) approval of its Pulsar-18 T3 peripheral self-expanding stent system. Full US...
New data on Biotronik’s Passeo-18 Lux DCB presented at LINC 2022
Biotronik announced the presentation of two studies on the performance of its drug-coated balloon (DCB) catheter Passeo-18 Lux at the Leipzig Interventional Course (LINC)...
Five-year data presented at Charing Cross reinforce safety of Passeo-18 Lux...
New long-term data presented at Charing Cross (CX) demonstrated the safety of BIOTRONIK’s Passeo-18 Lux paclitaxel drug-coated balloon (DCB) for the treatment of peripheral...
First patient enrolled in BIONETIC-I study of iliac artery treatment with...
Biotronik has announced the first patient enrolment in the BIONETIC-I study of the safety and efficacy of the Dynetic-35 cobalt chromium balloon-expandable stent system...
BIOPACT head-to-head non-inferiority randomised controlled trial completes enrolment
Biotronik is proud to announce the completion of enrolment of the investigator-initiated BIOPACT randomised controlled trial (RCT). This non-inferiority study evaluates the safety and...
Biotronik expands range of peripheral introducer sheaths
Biotronik has announced the expansion of the Fortress reinforced introducer sheath line, which is now available in 7- and 8Fr-compatible sizes in the USA...
BIOLUX AV trial demonstrates safety and efficacy of Passeo-18 Lux DCB...
Recent data from the investigator-initiated, randomised controlled trial (RCT), BIOLUX AV, showed that the treatment of patients with dysfunctional haemodialysis access with BIOTRONIK's Passeo-18...
Biotronik’s Passeo-35 Xeo PTA balloon catheter is now available in Europe
Biotronik today announced European market release of the Passeo-35 Xeo percutaneous transluminal angioplasty (PTA) balloon catheter. A company press release states that, compared to...
Dynetic-35 balloon-expandable cobalt chromium iliac stent system gains CE mark
Dynetic-35, the balloon-expandable cobalt chromium iliac stent system from Biotronik, is now commercially available in Europe. Indicated for the treatment of de novo or...
Biotronik appoint company’s first chief medical officer
David Hayes, former physician administrator at Mayo Clinic, Rochester, USA, has been appointed chief medical officer of Biotronik, a company press release reports.
"Our focus...
Proximo Medical named commercialisation partner for Biotronik’s PVI portfolio in select...
According to a press release, Proximo Medical has been announced as the commercial partner for Biotronik's peripheral vascular intervention (PVI) platform in select US markets.
Proximo Medical is...
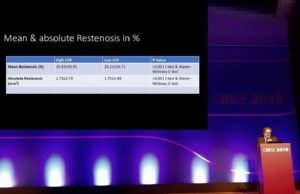
New data show high chronic outward force causes significantly more restenosis
Twelve-month results of the BIOFLEX COF trial have demonstrated that the implantation of stents with low chronic outward force (COF) was associated with less...
Biotronik expands peripheral portfolio with a new treatment tool for interventions...
Biotronik has launched the Carnelian support catheter, designed to improve the access for treatment of tortuous and highly calcified lesions. Carnelian support is indicated...
Passeo-18 Lux DCB remains safe and effective at two years with...
Two-year data on the Passeo-18 Lux drug-coated balloon (DCB; Biotronik) continue to validate its safety and effectiveness in intra-inguinal arteries. Gunnar Tepe from the...
Biotronik introduces the first tri-axial 4F low-profile stent system
Biotronik has presented the company's Pulsar-18 T3 stent system for the first time during the Leipzig Interventional Course (LINC; 22–24 January, Leipzig, Germany).
The Pulsar-18...
Encouraging results for Biotronik’s Passeo-18 Lux drug-coated balloon and Pulsar-18 bare...
Interim data presented at the 2017 Leipzig Interventional Course (LINC; 24-27 January, Leipzig, Germany) has demonstrated encouraging results for Biotronik’s dedicated lower limb intervention...